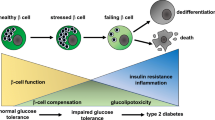
Abstract
Type 1 and type 2 diabetes are both diseases of insulin insufficiency, although they develop by distinct pathways. The recent surge in the incidence of type 2 diabetes and the chronic ailments confronted by patients with either form of the disease highlight the need for better understanding of β-cell biology. In this review, we present recent work focused on this goal. Our hope is that basic research being conducted in this and other laboratories will ultimately contribute to the development of methods for enhancing β-cell function and survival in the context of both major forms of diabetes. Our strategy for understanding the β-cell involves a multidisciplinary approach in which tools from the traditional fields of biochemistry, enzymology, and physiology are teamed with newer technologies from the fields of molecular biology, gene discovery, cell and developmental biology, and biophysical chemistry. We have focused on two important aspects of β-cell biology in our studies: β-cell function, specifically the metabolic regulatory mechanisms involved in glucose-stimulated insulin secretion, and β-cell resistance to immune attack, with emphasis on resistance to inflammatory cytokines and reactive oxygen species.
Similar content being viewed by others
References
Becker, T. C., Beltrandel Rio, H., Noel, R. J., Johnson, J. H, and Newgard, C. B. (1994) Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus: enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J. Biol. Chem. 269, 21234–21238.
Becker, T. C., Noel, R. J., Johnson, J. H., Lynch, R. M., Hirose, H., Tokuyama, Y., et al. (1996) Differential effects of overexpressed glucokinase and hexokinase I in isolated islets: evidence for functional segregation of the high and low Km enzymes. J. Biol. Chem. 271, 390–394.
Ferber, S., BeltrandelRio, H., Johnson, J. H., Noel, R. J., Cassidy, L. E., Clark, S., et al. (1994) GLUT-2 gene transfer into insulinoma cells confers both low and high affinity glucose-stimulated insulin release: relationship to glucokinase activity. J. Biol. Chem. 269, 11523–11529.
Asfari, M., Janjic, D., Meda, P., Li, G., Halban, P. A., and Wolheim, C. B. (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178.
Hohmeier, H. E., Mulder, H., Chen, G., Henkel-Reiger, R., Prentki, M., and Newgard, C. B. (2000) Isolation of INS-a-derived cell lines with robust KATP channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430.
MacDonald, M. J. (1990) Elusive proximal signals of β-cells for insulin secretion. Diabetes 29, 1461–1466.
Newgard, C. B. and Matschinsky, F. M. (2001) Regulation of insulin secretion from the endocrine pancreas, in Handbook of Physiology (Jefferson, J. and Cherrington, A., eds.), Oxford University Press, Oxford, UK.
MacDonald, M. J. (1981) High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J. Biol. Chem. 256, 8287–8290.
Lu, D., Mulder, H., Zhao, P., Burgess, S. C., Jensen, M. V., Kamzolova, S., et al. (2002) 13C isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion. Proc. Natl. Acad. Sci. U S A 99, 2708–2713.
MacDonald, M. J. (1982) Evidence for the malate aspartate shuttle in pancreatic islets. Arch. Biochem. Biophys. 213, 643–649.
Farfari, S., Schulz, V., Corkey, B., and Prentki, M. (2000) Glucose-regulated anaplerosis and cataplerosis in pancreatic β-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49, 718–726.
Maechler, P. and Wollheim, C. B. (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689.
MacDonald, M. J. and Fahien, L. A. (2000) Glutamate is not a messenger in insulin secretion. J. Biol. Chem. 275, 34025–34027.
Mulder, H., Lu, D., Finley, J., An, J., Cohen, J., Antinozzi, P., et al. (2001) Overexpression of a modified human malonyl-Co-A decarboxylase blocks the glucose-regulated increase in malonyl CoA level but has no impact on insulin secretion in INS1-derived (823/13) β-cells. J. Biol. Chem. 276, 6479–6484.
MacDonald, M. J. (1996) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets: further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 270, 20051–20058.
Newgard, C. B. (2002). While tinkering with the β-cell metabolic regulatory mechanisms and new therapeutic strategies. American Diabetes Association Lilly Lecture, 2001. Diabetes 51, 3141–3150.
Chen, G., Hohmeier, H. E., Gasa, R., Tran, V. V., and Newgard, C. B. (2000) Selection of insulinoma cell lines with resistance to interleukin-1β and γ-interferon-induced cytotoxicity. Diabetes 49, 562–570.
Rabinovitch, A., Suarez-Pinson, W., Strynadka, K., Ju, Q., Edelstein, D., Brownlee, M., et al. (1999) Transfection of human pancreatic islets with an anti-apoptotic gene (bcl-2) protects β-cells from cytokine-induced destruction. Diabetes 48, 1223–1229.
Dupraz, P., Rinsch, C., Pralong, W. F., Rolland, E., Zufferey, R., Trono, D., et al. (1999) Lentivirus-mediated Bcl-2 expression in βTC-tet cells improves resistance to hypoxia and cytokine-induced apoptosis while preserving in vitro and in vivo control of insulin secretion. Gene Therapy 6, 1160–1169.
Iwahashi, H., Hanafusa, T., Eguchi, Y., Nakajima, H., Miyagawa, J., Itoh, N., et al. (1996) Cytokine-induced apoptotic cell death in a mouse pancreatic β-cell line: inhibition by Bcl-2. Diabetologia 39, 530–536.
Tran, V. V., Chen, G., Newgard, C. B., and Hohmeier, H. E. (2003) Discrete and complementary mechanisms of protection of β-cells against cytokine-induced and oxidative damage achieved by bcl-2 overexpression and a cytokine selection strategy. Diabetes 52, 1423–1432.
Mandrup-Poulsen, T. (1996) The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39, 1005–1029.
Rabinovitch, A. (1993) An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Rev. 1, 215–240.
Corbett, J. A. and McDaniel, M. L. (1992) Does nitric oxide mediate autoimmune destruction of β-cells? Possible therapeutic interventions in IDDM. Diabetes 41, 897–903.
Eizirik, D. L., Flodstrom, M., Karlsen, A. E., and Welsh, N. (1996) The harmony of spheres: inducible nitric oxide synthase and related genes in pancreatic β-cells. Diabetologia 39, 875–890.
Schindler, C. and Darnell, J. E. (1995) Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Ann. Rev. Biochem. 64, 621–651.
Leaman, D. W., Leung, S., Li, X., and Stark, G. R. (1996) Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 10, 1578–1588.
Boehm, U., Klamp, T., Groot, M., and Howard, J. C. (1997) Cellular responses to interferon-γ. Ann. Rev. Immunol. 15, 749–795.
Chen, G., Hohmeier, H. E., and Newgard, C. B. (2001) Expression of the transcription factor STAT-1 alpha in insulinoma cells protects against cytotoxic effects of multiple cytokines. J. Biol. Chem. 276, 766–772.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newgard, C.B., Hohmeier, H.E., Lu, D. et al. Understanding of basic mechanisms of β-cell function and survival. Cell Biochem Biophys 40 (Suppl 3), 159–167 (2004). https://doi.org/10.1385/CBB:40:3:159
Issue Date:
DOI: https://doi.org/10.1385/CBB:40:3:159