Abstract
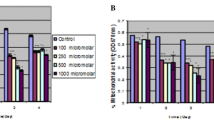
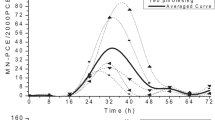
We are trying to understand individual differences in susceptibility to chromate toxicity by comparing three different lymphoblastic cell lines derived from three different individuals. We have compared the uptake of CrO 2−4 , the release of LDH from cells, the proliferation ability of the cells, and the DNA-protein crosslinks in these lymphoblastic cell lines exposed to chromate. We report here that one lymphoblastic cell line, GM0922B, appears to be considerably less sensitive than the other two cells lines to the cytotoxic effects of hexavalent chromium. The diminished sensitivity is almost twofold and can be accounted for by the decreased uptake of hexavalent chromium, which results in less lactate dehydrogenase release, and greater tolerance to chromate inhibition of cell proliferation and less DNA-protein crosslinking. This lower uptake of chromate combined with interindividual differences in extracellular Cr(VI) reducing capacity are probably the two most important determinants of genetic susceptibility to chromate toxicity.
Similar content being viewed by others
References
International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carciogenic Risk of Chemicals to Humans: Chromium, Nickel and Welding. IARC, Lyon, pp. 1–214 (1990).
M. D. Cohen, B. Kargacin, C. B. Klein, and M. Costa, Mechanisms of chromium carcinogenicity and toxicity, Crit. Rev. Toxicol. 23, 255–281 (1993).
M. Costa, Toxicity and carcinogenicity of Cr(VI) in animal models and humans, Crit. Rev. Toxicol. 27, 431–442 (1997).
S. Langer, One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports, Am. J. Ind. Med. 17, 189–215 (1990).
T. Norseth, The carcinogenicity of chromium and its salts, Br. J. Ind. Med. 43, 649–651 (1986).
P. Arslan, M. Beltrame, and A. Tomasi, Intracellular chromium reduction, Biochim. Biophys. Acta 931, 10–15 (1987).
K. W. Jennette, The role of metals in carcinogenesis: biochemistry and metabolism, Environ. Health Perspect. 40, 233–252 (1981).
A. G. Levis and V. Bianchi, Mutagenic and cytogenetic effect of chromium compounds, in Biological and Environmental Aspects of Chromium, S. Langard, ed., Elsevier Biomedicals, Amsterdam, pp. 177–208 (1982).
S. De Flora and K. E. Wetterhahn, Mechanisms of chromium metabolism and genotoxicity, Life Chem. Rep. 7, 169–244 (1989).
S. De Flora, M. Bagnasco, D. Serra, and P. Zanacchi, P. Genotoxicity of chromium compounds. A review, Mutat. Res. 238, 99–172 (1990).
M. Sugiyama, X. W. Wang, and M. Costa, Comparison of DNA lesions and cytotoxicity induced by calcium chromate in human, mouse, and hamster cell lines, Cancer Res. 46, 4547–4551 (1986).
M. Sugiyama, S. R. Patierno, O. Cantoni, and M. Costa, Characterization of DNA lesions induced by CaCrO4 in synchronous and asynchronous cultured mammalian cells, Mol. Pharmacol. 29, 606–613 (1986).
Z. Elisa, O. Poirot, O. Schneider, M. C. Daniere, F. Terzett, J. D. Guedenet, et al., Cellular uptake, cytotoxic and mutagenic effects of insoluble chromic oxide in V79 Chinese hamster cells, Mutat. Res. 169, 159–170 (1986).
B. L. Finley, B. D. Kerger, M. W. Katona, M. L. Gargas, G. C. Corbett, and D. J. Paustenbach, Human ingestion of chromium(VI) in drinking water: pharmacokinetics following repeated exposure, Toxicol. Appl. Pharmacol. 142, 151–159 (1997).
B. D. Kerger, B. L. Finley, G. E. Corbett, D. G. Dodge, and D. J. Paustenbach, Ingestion of chromium(VI) in drinking water by human volunteers: absorption, distribution, and excretion of single and repeated doses, J. Toxicol. Environ. Health 50, 67–95 (1997).
M. J. Tsapakos, T. H. Hamptom, and K. E. Wetterhahn, Chromium (VI)-induced DNA lesions and chromium distribution in rat kidney, liver, and lung, Cancer Res. 43, 5662–5667 (1983).
F. N. Henshaw, M. C. Little, D. J. Gawkrodger, et al., Keratinocytes cultured form normal adult skin show donor variability in their sensitivity to chromium, Br. J. Dermatol. 136, 455 (1997) (abstract).
F. N. Henshaw, B. W. Morris, and S. Mac Neil, Differentiation of normal human keratinocytes influences hexavalent chromium uptake and distribution and the ability of cells to withstand Cr(VI) cytotoxicity, Br. J. Dermatol. 141, 211–217 (1999).
G. Warren, P. Schultz, D. Bancroft, K. Bennett, E. H. Abbot, and S. Rogers, Mutagenicity of a series of hexacoordinate chromium (III) compounds, Mutat. Res. 90, 111–118 (1981).
J. Xu, G. J. Bubley, B. Detrick, L. J. Blankenship, and S. R. Patierno, Chromium(VI) treatment of normal human lung cells results in guanine-specific DNA polymerase arrest, DNA-DNA crosslinks and S-phaseblockade of cell cycle, Carcinogensis 17, 1511–1517 (1996).
A. Zhitkovich, A. Lukanova, T. Popov, E. Taioli, H. Cohen, and M. Costa, DNA-protein crosslinks in peripheral lymphocytes of individuals exposed to hexavalent chromium compounds, Biomarkers 1, 86–93 (1996).
M. Costa, A. Zhitkovich, M. Harris, D. Paustenbach, and M. Gargas, DNA-protein crosslinks produced by carious chemicals in culture human lymphoma cells, J. Toxicol. Environ. Health 50, 433–449 (1997).
A. Zhitkovich and M. Costa, A simple, sensitive assay to detect DNA-protein-crosslinks in intact cells and in vivo, Carcinogenesis 13, 1485–1489 (1992).
A. Zhitkovich, V. Voitkun, and M. Costa, Formation of amino-acid-DNA complex by trivalent chromium in vitro: importance of trivalent chromium and the phosphate group, Biochemistry 35, 7275–7282 (1996).
T. P. Coogan, K. S. Squibb, J. Motz, P. L. Kinney, and M. Costa, Distribution of chromium within cells of the blood, Toxicol. Appl. Pharmacol. 108, 157–166 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costa, M., Kluz, T., Salnikow, K. et al. Comparison of the cytotoxicity, cellular uptake, and DNA-protein crosslinks induced by potassium chromate in lymphoblast cell lines derived from three different individuals. Biol Trace Elem Res 86, 11–22 (2002). https://doi.org/10.1385/BTER:86:1:11
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:86:1:11