Abstract
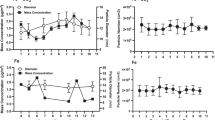
Neonatal female and male rats were exposed to airborne manganese sulfate (MnSO4) during gestation and postnatal d 1–18. Three weeks post-exposure, rats were killed and we assessed biochemical end points indicative of oxidative stress in five brain regions: cerebellum, hippocampus, hypothalamus, olfactory bulb, and striatum. Glutamine synthetase (GS) protein levels, metallothionein (MT) and GS mRNA levels, and total glutathione (GSH) levels were determined for all five regions. Overall, there was a statistically significant effect of manganese exposure on decreasing brain GS protein levels (p=0.0061), although only the highest dose of manganese (1 mg Mn/m3) caused a significant increase in GS messenger RNA (mRNA) in both the hypothalamus and olfactory bulb of male rats and a significant decrease in GS mRNA in the striatum of female rats. This highest dose of manganese had no effect on MT mRNA in either males or females; however, the lowest dose (0.05 mg Mn/m3) decreased MT mRNA in the hippocampus, hypothalamus, and striatum in males. The median dose (0.5 mg Mn/m3) led to decreased MT mRNA in the hippocampus and hypothalamus of the males and olfactory bulb of the females. Overall, manganese exposure did not affect total GSH levels, a finding that is contrary to those in our previous studies. Only the cerebellum of manganese-exposed young male rats showed a significant reduction (p<0.05) in total GSH levels compared to control levels. These data reveal that alterations in biomarkers of oxidative stress resulting from in utero and neonatal exposures of airborne managanese remain despite 3 wk of recovery; however, it is important to note that the doses of manganese utilized represent levels that are 100-fold to a 1000-fold higher than the inhalation reference concentration set by the US Environmental Protection Agency.
Similar content being viewed by others
References
A. W. Dobson, S. Weber, D. C. Dorman, L. K. Lash, K. M. Erikson, and M. Aschner, Inhaled manganese sulfate and measures of oxidative stress in rat brain. Biol. Trace Element Res. 93, 113–126 (2003).
S. Weber, D. C. Dorman, L. H. Lash, K. Erikson, K. E. Vrana, and M. Aschner Effects of managanese (Mn) on the developing rat brain: oxidative-stress related endpoints. Neurotoxicology 23, 169–175 (2002).
K. M. Erikson, D. C. Dorman, L. H. Lash, A. W. Dobson, and M. Aschner. Airborne manganese exposure differentially affects endpoints of oxidative stress in an age and sex-dependent manner. Biol Trace Element Res. 100, 49–62 (2004).
L. S. Hurley and C. L. Keen, Manganese,. in Trace Elements in Human Health and Animal Nutrition, E. Underwood and W. Mertz, eds., Academic, New York, pp. 185–223 (1987).
M. Aschner, K. M. Erikson, and D. C. Dorman, Manganese dosimetry: species differences and implications for neurotoxicity. Crit. Rev. Toxicol. 35, 1–32 (2005).
ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Managanese, US Department of Health and Human Services Public Health Service. Available at http://www.atsdr.cdc.gov/toxprofiles/tp151.html (accessed September 2000).
D. Mergler, G. Huel, R. Bowler, et al., Nervous system dysfunction among workers with long-term exposure to managenese. Environ. Res. 64, 151–180 (1994).
P. K. Pal, A. Samii, and D. B. Calne, Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20, 227–238 (1999).
E. D. Pellizzari, C. A. Clayton, C. E. Rodes, et al., Particulate matter and manganese exposures in Indianapolis, Indiana, J. Exp. Anal. Environ. Epidemiol. 11, 423–440 (2001).
M. Aschner, Manganese neurotoxicity and oxidative damage, in Metals and Oxidative Damage in Neurological Disorders, J. R. Connor, ed., Plenum, New York, pp. 77–93 (1997).
W. N. Sloot, J. Kort, J. F. Koster, L. E. A. DeWit, and J. B. P. Gramsbergen, Manganese-induced hydroxyl radical formation in rat striatum is not attenuated by dopamine depletion or iron chelation in vivo. Exp. Neurol. 138, 236–245 (1996).
P. Galvani, P. Fumagalli, and A. Santagostino, Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharmacol. 293, 377–383 (1995).
C. E. Gavin, K. K. Gunter, and T. E. Gunter, Manganese and calcium transport in mitochondria: implications for managanese toxicity. Neurotoxicology 20, 445–453 (1999).
F. S. Archibald and C. Tyree, Manganese poisoning and the attack of trivalent managanese upon catecholamines. Arch. Biochem. Biophys, 256, 638–650 (1987).
S. F. Ali, H. M. Duhart, G. D. Newport, G. W. Lipe, and W. Slikke, Mangenese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration, 4, 329–334 (1995).
J. Y. Chen, G. C. Tsao, Q. Zhao and W. Zheng, Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe−S] containing enzymes. Toxicol. Appl. Pharmacol. 175, 160–168 (2001).
K. K. Gunter, L. M. Miller, M. Aschner, et al., XANEs spectroscopy: a promising tool for toxicology: a tutorial. Neurotoxicology 23, 127–146 (2002).
D. HaMai A. Campbell, and S. C. Bondy, Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radical Biol. Med. 31, 763–768 (2001).
D. C. Dorman, B. E. McManus, M. W. Marshall, R. A. James, and M. F. Struve, Old age and gender influence the pharmacokinetics of inhaled managanese sulfate and manganese phosphate in rats. Toxicol. Appl. Pharmacol. 197, 113–124 (2004).
V. Barbu and F. Dautry, Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 17, 7115 (1989).
M. W. Fariss and D. J. Reed, High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives, Methods Enzymol. 143, 101–109 (1987).
L. H. Lash and J. J. Tokarz, Oxidative stress in isolated rat renal proximal and distal tubular cells, Am. J. Physiol 259, F338-F347 (1990).
L. H. Lash and E. B. Woods, Cytotoxicity of alkylating agents in isolated rat kidney proximal and distal tubular cells, Arch. Biochem. Biophys. 286, 46–56 (1991).
J. M. Davis, Inhalation health risks and manganese: an EPA perspective, Neurotoxicology 20, 511–518 (1999).
D. C. Dorman, A. M. McElveen, M. W. Marshall, et al., Maternal-fetal distribution of manganese in the rat following inhalation exposure to manganese sulfate, Neurotoxicology, in press.
D. C. Dorman, A. M. McElveen, M. W. Marshall, et al., Tissue manganese concentrations in lactating rats and their offspring following combined in utero and lactation exposure to inhaled manganese sulfate, Toxicol. Sci. 84, 12–21 (2005)
C. L. Keen, J. G. Bell, and B. Lonnerdal, The effect of age on manganese uptake and retention from milk and infant formulas in rats, J. Nutr. 116(3), 395–402 (1986).
A. Martinez-Hernandez, K. P. Bell, and M. D. Norenberg, Glutamine synthetase: glial localization in the brain, Science 195, 1356–1358 (1977).
U. Sonnewald, N. Westergaard, and A. Schousboe, Glutamate transport and metabolism in astrocytes, Glia 21, 56–63 (1997).
E. R. Stadtman, Protein oxidation and aging, Science 257, 1220–1224 (1992).
M. Aschner, The functional significance of brain metallothioneins, FASEB J. 10, 1129–1136 (1996).
S. Hussain, W. Slikker, Jr., and S. F. Ali, Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection, Neurochem. Int. 29, 145–152 (1996).
M. Kondoh, Y. Inoue, S. Atagi, et al., Specific induction of metallothionein synthesis by mitochondrial oxidative stress, Life Sci. 69., 2137–2146 (2001).
A. Meister and M. E. Anderson, Glutathione, Annu. Rev. Biochem. 52, 711–760 (1983).
M. S. Desole, G. Esposito, R. Mighelli, et al., Cellular defence mechanisms in the striatum of young and aged rats subchronically exposed to manganese, Neuropharmacology 34, 289–295 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Erikson, K.M., Dorman, D.C., Lash, L.H. et al. Persistent alterations in biomarkers of oxidative stress resulting from combined in Utero and neonatal manganese inhalation. Biol Trace Elem Res 104, 151–163 (2005). https://doi.org/10.1385/BTER:104:2:151
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:104:2:151