Abstract
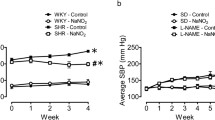
The aim of this study was to determine the levels of tissue and blood zinc (Zn), copper (Cu), magnesium (Mg) in nitric oxide (NO) synthase blockade-induced hypertension. A group of albino rats received a NO synthase inhibitor, N G-nitro-l-arginine-methyl ester (l-NAME, 60 mg/kg/d) in their drinking water for 21 d. l-NAME intake caused a progressive rise in this group’s resting mean arterial blood pressure compared to a control group (p<0.01). There were no differences between the groups with regard to tissue and blood levels of Zn or Cu; however, Mg concentrations were significantly lower in the hypertensive rats’ erythrocytes (20.2% reduction from control levels), cerebral cortex (17.0%), heart (9.1%), renal cortex (12%), renal medulla (16.7%), and in the tissues of the caval vein (23.7%), mesenteric artery (29.8%), renal artery (18.4%), and renal vein (22.1%). There were no significant Mg concentration changes in the hypertensive group’s plasma, cerebellum, liver, duodenum, or aortal tissue. These findings suggest that Mg depletion may play a role in the blood pressure rise that occurs in the model of chronic NO synthase inhibition-induced hypertension.
Similar content being viewed by others
References
J. Navarro, A. Sanchez, J. Saiz, L. M. Ruilope, J. Garcia-Estan, C. Romero, et al., Hormonal, renal, and metabolic alterations during hypertension induced by chronic inhibition of NO in rats, Am. J. Physiol. 267, R1516-R1521 (1994).
M. O. Riberio, E. Antunes, G. Nucci, S. M. Lovisolo, and R. Zatz, Chronic inhibition of nitric oxide synthesis, a new model of arterial hypertension, Hypertension 20, 298–303 (1992).
Jr R. D. Manning, L. Hu, and J. F. Reckelhoff, Role of nitric oxide in arterial pressure and renal adaptations to long-term changes in sodium intake, Am. J. Physiol. 272, R1162-R1169 (1997).
G. Frithz and G. Ronquist, Increased red cell content of Zn2+ in essential hypertension, Acta Med. Scand. 205, 647–649 (1979).
J. G. Henrotte, M. Santarromana, G. Franck, and R. Bourdon, Blood and tissue zinc levels in spontaneously hypertensive rats, J. Am. Coll. Nutr. 9, 340–343 (1990).
G. Vivoli, P. Borella, P. Bergomi, and G. Fantuzzi, Zinc and copper levels in serum, urine, and hair of humans in relation to blood pressure, Sci. Total Environ. 66, 55–64 (1987).
A. Berthelot, C. Luthringer, and A. Exinger, Trace elements during the development of hypertension in the spontaneously hypertensive rat, Clin. Sci. 72, 515–518 (1987).
M. S. Clegg, F. Ferrel, and C. L. Keen, Hypertension-induced alterations in copper and zinc metabolism in Dahl rats, Hypertension 9, 624–628 (1987).
S. Wallach and R. L. Verch, Tissue magnesium in spontaneously hypertensive rats, Magnesium 5, 33–38 (1986).
P. Laurant, J. P. Kantelip, and A. Berthelot, Dietary magnesium supplementation modifies blood pressure and cardiovascular function in mineralocorticoid-salt hypertensive rats but not in normotensive rats, J. Nutr. 125, 830–841 (1995).
H. Karppanen, Epidemiologic evidence for considering magnesium deficiency as a risk factor for cardiovascular diseases, Magnesium Bull. 12, 80–86 (1990).
I. Kaputlu, G. Sadan, B. Karayalcin, and A. Boz, Beneficial effects of pentoxifylline on cyclosporine-induced nephrotoxicity, Clin. Exp. Pharmacol. Physiol. 24, 365–369 (1997).
B. L. Vallee and K. H. Falchuk, The biochemical basis of zinc physiology, Physiol. Rev. 73, 79–118 (1993).
G. Leblondel and P. Allain, Altered element concentrations in tissues of spontaneously hypertensive rats, Biomed. Pharmacother. 42, 121–129 (1988).
N. Saito, G. C. Abbu, Y. Konishi, S. Nishiyama, and T. Okada, Magnesium, calcium and trace elements in spontaneously hypertensive rats, Clin. Exp. Pharmacol. Physiol. 22(Suppl. 1), S212-S214 (1995).
J. G. Henrotte, G. Franck, M. Santarromana, and R. Bourdon, Tissue and blood magnesium levels in spontaneously hypertensive rats, at rest and stressful conditions, Magnesium Res. 4, 91–96 (1991).
R. M. Touyz, F. J. Milne, and S. G. Reinach, Intracellular Mg2+, Ca2+, Na2+ and K+ in platelets and erythrocytes of essential hypertension patients: relation to blood pressure. Clin. Exp. Hypertens. 14, 1189–1209 (1992).
K. Kisters, M. Tepel, C. Spieker, K. H. Dietl, M. Barenbrock, K. H. Rahn, et al., Decreased cellular Mg2+ concentrations in a subgroup of hypertensives-cell models for the pathogenesis of primary hypertension. J. Hum. Hypertens. 11, 367–372 (1997).
L. M. Resnick, R. K. Gupta, and J. H. Laragh, Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc. Natl. Acad. Sci. USA 81, 6511–6515 (1984).
S. E. Kjeldsen, O. M. Sejersted, P. Frederichsen, P. Leren, and I. K. Eide, Increased erythrocyte magnesium content in essential hypertension, Scand. J. Clin. Lab. Invest. 50, 395–400 (1990).
R. K. Rude, Magnesium metabolism and deficiency, Endocrinol. Metab. Clin. North Am. 22, 377–395 (1993).
T. Nakamura, A. M. Alberola, F. J. Salazar, Y. Saito, T. Kurashina, J. P. Granger, et al., Effects of renal perfusion pressure on renal interstitial hydrostatic pressure and Na+ excretion: role of endothelium-derived nitric oxide, Nephron 78, 104–111 (1998).
C. de Rouffignac, B. Mandon, M. Wittner, and A. di Stefano, Hormonal control of renal magnesium handling, Miner. Electrolyte Metab. 19, 226–231 (1993).
F. E. Kaiser, M. Dorighi, J. Muchnick, J. E. Morley, and P. Patrick, Regulation of gonadotropins and parathyroid hormone by nitric oxide, Life Sci. 59, 987–992 (1996).
A. Zanchi, N. C. Schaad, M. C. Osterheld, E. Grouzmann, J. Nussberger, H. R. Brunner, et al., Effect of chronic NO synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system, Am. J. Physiol. 268, H2267-H2273 (1995).
M. Giordino, M. Vermeulen, A. S. Trevani, G. Dran, G. Andonegui, and J. R. Geffner, Nitric oxide synthase inhibitors enhance plasma levels of corticosterone and ACTH, Acta Physiol. Scand. 157, 259–264 (1996).
M. Haluzik, J. Nedvidkova, V. Kopsky, J. Jahodova, B. Horejsi, and V. Schreiber, The changes of the thyroid function and serum testosterone levels after long-term l-NAME treatment in male rats, J. Endocrinol. Invest. 21, 234–238 (1998).
Y. Erlich and T. Rosenthal, Chronic hypertension leads to hyperinsulinemia in Sprague-Dawley rats treated with nitric oxide synthase inhibitor, Am. J. Hypertens. 11, 1129–1133 (1998).
R. M. Mclean, Magnesium and its therapeutic uses-a review, Am. J. Med. 96, 63–76 (1994).
B.M. Altura, B.T. Altura, A. Gebrewold, H. Ising, and T. Ghünter, Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and micro-circulatory changes in situ, Science 223, 1315–1317 (1984).
A. Zhang, B. T. Altura, and B. M. Altura, Endothelial cells are required for inhibition of contractile responses induced by reduction in extracellular magnesium and sodium ions in rat aortic smooth muscle. Microcirc. Endothelium Lymphatics 6, 427–435 (1990).
C. Szabo, V. Berczi, F. Schneider, A. G. Kovach, and E. Monos, Role of endothelium in the response of the vein wall to magnesium withdrawal, Pflugers Arch. 420, 140–145 (1992).
D. D. Ku and H. S. Ann, Differential effects of magnesium on basal and agonist-induced EDRF relaxation in canine arteries, J. Cardiovasc. Pharmacol. 17, 999–1006 (1991).
I. T. Mak, A. M. Komarov, T. L. Wagner, R. E. Stafford, B. F. Dickens, and W. B. Weglicki, Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss, Am. J. Physiol. 271, C385-C390 (1996).
P. Laurant and A. Berthelot, Influence of endothelium on Mg2+-induced relaxation in noradrenaline-contracted aorta from DOCA-salt hypertensive rat, Eur. J. Pharmacol. 258, 167–172 (1994).
P. Laurant, S. Robin, and A. Berthelot, Magnesium deficiency increases vasoconstrictor activity without affecting blood pressure of aged spontaneously hypertensive rats, Magnes. Res. 10, 107–117 (1997).
M. Adachi, Y. Nara, M. Mano, and Y. Yamori, Effect of dietary magnesium supplementation on intralymphocytic free calcium and magnesium in stroke-prone spontaneously hypertensive rats, Clin. Exp. Hypertens. 16, 317–326 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kemal Şentürk, Ü., Kaputlu, İ., Gündüz, F. et al. Tissue and blood levels of zinc, copper, and magnesium in nitric oxide synthase blockade-induced hypertension. Biol Trace Elem Res 77, 97–106 (2000). https://doi.org/10.1385/BTER:77:2:97
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:77:2:97