Abstract
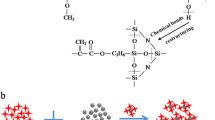
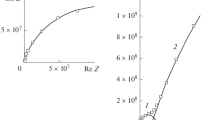
Solvent-free siloxane-modified epoxy coatings were developed by the interpenetrating technique using epoxy resin as base (DGEBA, GY 250, Ciba-Geigy) and hydroxyl-terminated polydimethylsiloxane as modifier (commercially known as silicone) with γ-aminopropyltriethoxysilane as cross-linker, dibutyltindilaurate as catalyst, and 25% zinc powder as additive. Hexamethylenediamine (Aldrich) and polyamidoamine (HY 840, Ciba-Geigy) were used as curatives for the siliconized epoxy coatings containing 25% zinc powder. The corrosion-resistant behavior of these coating systems is assessed by electrochemical methods such as electrochemical potential measurements, potentiodynamic polarization, and electrochemical impedance spectroscopic methods. Based on the results obtained from the electrochemical potential measurements for epoxy and siliconized epoxy coating systems, few samples, namely AX4 and BX4, have been found to be the best corrosion-resistant coating systems, and they are used for potentiodynamic polarization measurements, electrochemical impedance, and salt-spray tests. The experimental results reveal that the siloxane (10%) modified epoxy coating system (AX4) with 25% zinc powder cured by hexamethylenediamine offers the maximum corrosion protection to the steel surface rather than the polyamidoamine-cured system (BX4). The better protective action offered by the coating system (AX4) is mainly imparted by the reaction of aliphatic amine hydrogens with oxirane groups of the epoxy resin, which gives coating films with a high cross-link density. The observation is further supported by a capacitive behavior in the Nyquist plot and no spreading of visible corrosion product in the salt-spray test.
Similar content being viewed by others
References
F. Theiler: “The Rust Preventing Mechanism of Zinc Dust Paints,” Corros. Sci., 1974, 14(8), pp. 405–14.
L.H. Sperling and D.W. Friedmann: “Synthesis and Mechanical Behavior of Interpenetrating Polymer Networks: Poly(ethyl acrylate) and Polystyrene,” J. Polym. Sci. A. Polym. Chem., 1969, 7, pp. 425–27.
L.H. Sperling: Recent Advances in Polymer Blends, Grafts and Block, Plenum Press, New York, NY, 1974.
L.H. Sperling: Interpenetrating Polymer Networks and Related Materials, Plenum Press, New York, NY, 1981.
R.F. Boyer: History of Polymer Science and Technology, R.B. Seymour, ed., Marcel Dekker, New York, NY, 1982.
K.H. Hsieh and J.L. Han: “Graft Interpenetrating Polymer Networks of Polyurethane Epoxy. I. Mechanical Behavior,” J. Polym. Sci. B, Polym. Phys., 1990, 28, pp. 623–30.
K.H. Hsieh and J.L. Han: “Graft Interpenetrating Polymer Networks of Polyurethane Epoxy. II. Toughening Mechanism,” J. Polym. Sci. B, Polym. Phys., 1990, 28, pp. 783–94.
P.H. Sung and C.Y. Lin: “Polysiloxane Modified Epoxy Polymer Networks—I. Graft Interpenetrating Polymeric Networks,” Eur. Polym. J., 1997, 33, pp. 903–06.
J.N. Sultan and F.J. McGarry: “Effect of Rubber Particle Size on Deformation Mechanism on Glassy Epoxy,” J. Appl. Polym. Sci., 1973, 13, pp. 29–33.
A.J. Kinloch., S.J. Shaw, and D.L. Hunston: “Deformation and Fracture Behavior of Rubber Toughened Epoxy. 2. Failure Criteria,” Polymer, 1983, 32, pp. 1341–44.
A.F. Yee and R.A. Pearson: “Toughening Mechanism in Elastomer Modified Epoxies Part I. Mechanical Studies,” J. Mater. Sci., 1986, 21, pp. 2462–74.
W. Noll: Chemistry and Technology of Silicones, Academic Press, New York, NY, 1969.
T.V. Thanikai Velan: Studies on Synthesis and Characterization of E-Glass/Kevlar 49 Reinforced Siliconized Epoxy Matrix Composites for High Performance Engineering Applications, Ph.D. Thesis, Anna University, Chennai, India, 1997.
I. Sekine: “Recent Evaluation of Corrosion Protection of Paint Films by EIS,” Prog. Org. Coatings, 1997, 31, pp. 73–80.
E.V. Schmid: Exterior Durability of Organic Coatings, FMJ International Publications Limited, Redhill, Surrey, England, 1988, pp. 28, 29, 112.
E. Van Westing: Determination of Coating Performance with Impedance Measurements, Ph.D. Thesis, Delft University, The Netherlands, 1992.
I. Suzuki: Corrosion-Resistant Coating Technology, P.A. Schweitzer, New York, NY, 1989.
G.W. Walter: Corros. Sci., 26(9), p. 681.
S. Narain, N. Bonanos, and M.G. Hocking: JOCCA, 1983, 66, p. 43.
J.R. Scully: “Electrochemical Impedance of Organic Coated Steel,” J. Electrochem. Soc., 1989, 136(4), p. 979.
H. Lee and K. Neville: Handbook of Epoxy Resins, McGraw-Hill Book Company, New York, NY, 1967.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, S.A., Alagar, M. & Mohan, V. Studies on corrosion-resistant behavior of siliconized epoxy interpenetrating coatings over mild steel surface by electrochemical methods. J. of Materi Eng and Perform 11, 123–129 (2002). https://doi.org/10.1361/105994902770344178
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1361/105994902770344178