Study of Mesoionic Compounds: Synthesis and Pharmacological Evaluation of Several 2-[{(4-Substituted-1-sulphonyl) Sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles as Antimicrobial, Insecticidal and Antihelmintic Agents
Ranjana Dubey1, Nidhi Chaudhary2, Ravindra Kumar3 and Hament Panwar3*
1Department of Chemistry, S.R.M. University, Modinagar-201204, Ghaziabad, U.P., India
2Department of Chemistry, M.I.E.T., Meerut-250001, U.P., India
3Department of Chemistry, Neelkanth Institute of Technology, Modipuram-250110, Meerut, U.P., India
DOI : http://dx.doi.org/10.13005/ojc/300134
Article Received on :
Article Accepted on :
Article Published : 06 Feb 2014
Antimicobial; insecticidal; antihelmintic; sydnones; indoles.
Download this article as:| Copy the following to cite this article: Dubey1 R , Chaudhary2 N , Kumar3 R ,Panwar3 H *. Study of Mesoionic Compounds: Synthesis and Pharmacological Evaluation of Several 2-[{(4-Substituted-1-sulphonyl) Sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles as Antimicrobial, Insecticidal and Antihelmintic Agents. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Dubey1 R , Chaudhary2 N , Kumar3 R ,Panwar3 H *. Study of Mesoionic Compounds: Synthesis and Pharmacological Evaluation of Several 2-[{(4-Substituted-1-sulphonyl) Sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles as Antimicrobial, Insecticidal and Antihelmintic Agents. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2031 |
INTRODUCTION
An enormous amount of research on synthetic and pharmacological studies of sydnones has been reported during the last few decades1. Mesoionic compounds are non-benzenoid heterocycles containing different combination of hetero atoms. Sydnones being mesoionic compounds, are 1, 2, 3-oxadiazolium-5-olates and their chemistry has been widely studied by the scientific workers2-3 due to their electronic and versatile biological profile. Literature is evident to reveal that sydnone derivatives are most important member of the mesoionic category of compounds. With few exceptions, sydnones are stable compounds and display significant polarity. Sydnones are reported to posses anticonvulsant4, antitumor5, diuretic6, antimicrobial7-8, hypotensive9, anticancer10, analgesic11, insecticidal12 and antihelmintic13 activities. On the other hand, indole and its analogs showed versatile biological spectrum as anti-inflammatory14-18, anticonvulsant19, antitumor20, antimicrobial21, antibacterial22-23, antifungal24 and antihelmintic25. In continuation of research work26-27, we focused our efforts on the synthesis of 2-[{(4-Substituted-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles 4a-f starting from 2-amino-1, 3, 4-thiadiazino (6, 5-b) indole (Scheme-1).
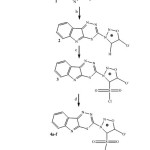 |
Scheme 1: Click here to View Scheme |
Hence all these observation prompted us to synthesis some novel derivatives of indoles bearing syndnone moiety. It was interesting to study the biological behaviours of various substituted sydnones.
RESULTS AND DISCUSSION
Synthetic strategy has been outlined in Scheme-1. Compound 1 and 2 were synthesised by our earlier reported method27. The condensation reaction of compound 2 and chloro sulphonic acid in presence of catalytic amount of phosphorous pentoxide yielded the compound 3. The formation of compound 3 was evidenced by the disappearance of singlet in 1H-NMR spectra and IR spectral band at δ 4.69 ppm and 3106 cm-1 respectively. It was further evidenced by the appearance of IR spectral band at 1180 cm-1 due to the SO2 group. The desired derivatives, 4a-f were obtained by the reaction of compound 3 and secondary amines. In 1H-NMR spectra compounds 4, the peaks were observed at δ 2.02-2.51 due to hydrogens of morpholine ring. Presence of absorption band at 1460, 1120 for C-O-C of morpholine ring cleared the synthesis of derivatives 4a-f. Scheme-2 explored the general mass fragmentation of 2-[{(4-morpholino-1-sulphonyl) sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles 4a. In the mass spectra of compound 4a, the molecular ion peak 420 [M+] (15%) also confirmed the formation of the sydnonyl substituted indoles. The molecular, base peak and other peak with their relative intensities were summarised in Table-4. The mass spectral study of sydnonyl substituted indoles publicized that parent compound 4a cleaved and released gradually fragment radicals and neutrals by following two routes viz route A and route B. Route A produced two daughter ions [a]+ and [b]+ while route B also produced two daughter
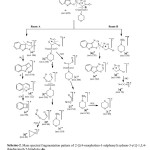 |
Scheme 2: Click here to View Scheme |
ions [n]+ and [o]+. Ion [a]+ releases N2 radical to give ion [c]+ which further expel out S radical to yield quinolinium ion [d]+. Quinolinium ion28 [d]+ splits to give ion [e]+. In ion [b]+, sydnonyl ringruptures similarly to that of other pattern of sydnone derivatives29 to yield two ions, ion [f]+ and [j]+. Ion [f]+ further cleaved into ions [g]+ and [s]+ . Ion [g]+ transformed into ions [h]+ and[i]+. Ion [j]+ breaks down to give ions [k]+ and[l]+ and SO2 release converted ion [l]+ into ion [m]+. Ions [p]+ and[q]+ were obtained by the splitting of ion [n]+. Expulsion of NO radical from ion [q]+ furnish ion [r]+
Table-1: Mass spectral data 2-[{(4-Morpholino-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles 4a.
|
Selected ions |
[M]+ |
[a]+ |
[b]+ |
[c]+ |
[d]+ |
[e]+ |
[f]+ |
[g]+ |
[h]+ |
[i]+ |
| m/z | 420 | 186 | 234 | 154 | 122 | 96 | 233 | 84 | 40 | 204 |
| Relative intensity (%) | 15 | 46 | 37 | 50 | 40 | 61 | 41 | 64 | 12 | 30 |
| Selected ions | [j]+ | [k]+ | [l]+ | [m]+ | [n]+ | [o]+ | [p]+ | [q]+ | [r]+ | |
| m/z | 54 | 150 | 86 | 270 | 150 | 162 | 108 | 78 | 150 | |
| Relative intensity (%) | 22 | 79 | 80 | 43 | 79 | 100 | 25 | 13 | 79 |
Pharmacology
Compounds 3 and 4a-f were evaluated for antimicrobial, insecticidal and antihelmintic activity (Table-2, 3 & 4).
Antimicrobial screening
All the newly synthesized compounds 3 and 4a-f were screened for their antibacterial and antifungal activity. All the bacterial as well as fungal strains were clinical isolates, identified
with conventional morphological and biochemical methods. The microorganisms employed antibacterial studies were Staphylococcus aureus, E. coli and Klabsiella pneumoniae. Disk diffusion method30-31 was used for determination of the preliminary antibacterial activity. Disks measuring 6.25 mm in diameter were punched from Whatman no. 1 filter paper. Batches of 100 disks were dispensed to each screw-capped bottle and sterilized by dry heat at 140 °C for an hour. The test compounds were prepared with different concentrations using DMF. One milliliter containing 100 times the amount of chemical in each disk was added to each bottle, which contained 100 disks. Disks of each concentration were for placed in triplicate in nutrient agar medium seeded with fresh bacteria separately. The incubation was carried out at 37 °C for 24 h. Ampicillin trihydrate and fluconazole were used as standard drugs. Solvent and growth controls were kept and zones of inhibition were noted. The bacterial inhibition values (mm) of the tested compounds against the tested bacterial strains are recorded in Table 2. On the other hand, the newly prepared compounds were screened for their in vitro antifungal activity against Aspergillus fumigatus (plant isolate), Candida albicans and Candida glabrata, in DMF by the serial plate dilution method32-33. Sabouraud’s agar media were prepared by dissolving peptone (1 g), D-glucose (4 g), and agar (2 g) in distilled water (100 ml) and adjusting the pH to 5.7. Normal saline was used to make a suspension of the spore of fungal strain for lawning. A loopful of particular fungal strain was transferred to 3 ml saline to get a suspension of the corresponding species. Agar media (20 ml) was poured into each petri dish. Excess suspension was decanted and the plates were dried by placing in an incubator at 37 °C for 1 h. Using an agar punch wells were made into each well labelled. A control was also prepared in triplicate and maintained at 37 °C for 3–4 days. Antifungal activity was determined by measuring the diameter of the inhibition zone. The fungicidal inhibitory (mm) values of the tested compounds against the tested fungal species are recorded in
Table-2: Antimicrobial screening of 2-[(4-Sulphoyl chloride)sydon-3-yl]-1,3,4-thiadiazino(6,5-b) indoles 3 and 2-[{(4-substituted-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b) indoles 4a-f.
|
Compound |
Antibacterial activity (mm) |
Antifungal activity (mm) |
||||
|
S. aureus |
E. coli |
K. pneumoniae |
A. fumigatus |
C. albicans |
C. glabrata |
|
|
3. |
– |
– |
5 |
– |
– |
– |
|
4a. |
10 |
15 |
20 |
16 |
10 |
12 |
|
4b. |
– |
6 |
10 |
8 |
6 |
– |
|
4c. |
– |
– |
8 |
6 |
– |
6 |
|
4d. |
– |
6 |
6 |
10 |
6 |
8 |
|
4e. |
5 |
10 |
10 |
8 |
6 |
10 |
|
4f. |
– |
6 |
8 |
14 |
– |
8 |
|
Ampicillin trihydrate |
16 |
16 |
20 |
– |
– |
– |
|
Fluconazole |
– |
– |
– |
20 |
15 |
15 |
|
DMF (control) |
– |
– |
– |
– |
– |
– |
Insecticidal activity
Periplaneta americana was taken for insecticidal study and 1 and 2% solutions of the derivatives 3 and 4a-f were injected in the abdominal of P. americana with the help of micro syringe. The time of death had been noted as KD (Knock promising to moderate activity) value. Cypermethrin was used as standard drug. At the time of death the antennae of P. americana became motionless, the appendages shrunk and folded towards the ventral side and cockroach lay dorsally34
Table-3: Insecticidal activity of 2-[(4-Sulphoyl chloride)sydon-3-yl]-1,3,4-thiadiazino(6,5-b)indoles 3 and 2-[{(4-substituted-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles 4a-f at two different concentrations (KD value in min.).
|
Comp. |
Time [min.] |
|
|
1% |
2% |
|
|
3. |
28 |
22 |
|
4a. |
8 |
6 |
|
4b. |
15 |
8 |
|
4c. |
18 |
12 |
|
4d. |
14 |
8 |
|
4e. |
12 |
10 |
|
4f. |
19 |
12 |
|
Cypermethrin |
7 |
5 |
Anthelmintic activity
Indian adult earthworms (Pheretima posthuma) were collected from moist soil and washed with normal saline to remove all faecal matter and used for the anthelmintic activity. All the synthesized derivatives 3 and 4a-f were dissolved in minimum amount of DMF and the volume adjusted to 10 ml with saline water. All solutions of synthesized derivatives and drugs solutions were freshly prepared. Groups of six earthworms were released into desired formulations and the paralytic and lethal time noted. Albendazole was used as standard drug. Observations were made for the time taken for paralysis and death of individual worms. Paralysis was said to occur when the worms did not receive even in normal saline. Death was concluded when the worms lost their motility followed by fading away of their body colour35-38
Table-4: Anthelmintic activity of 2-[(4-Sulphoyl chloride)sydon-3-yl]-1,3,4-thiadiazino(6,5-b) indoles 3 and 2-[{(4-substituted-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indoles 4a-f against Pheretima posthuma (paralytic and lethal time in min.).
|
Comp. |
Paralytic time (min.) |
Lethal time (min.) |
|
3. |
12 |
20 |
|
4a. |
6 |
10 |
|
4b. |
18 |
24 |
|
4c. |
13 |
20 |
|
4d. |
10 |
15 |
|
4e. |
14 |
18 |
|
4f. |
12 |
16 |
|
Albendazole |
5 |
8 |
EXPERIMENTAL
All the chemicals used for the preparation of desired derivatives, were obtained from Sisco Research Laboratories (SRL), Mumbai, India; Qualigen Fine Chemicals, Mumbai, India; E. Merck Ltd., New Delhi, India. The reference drugs ampicillin trihydrate, fluconazole, cypermethrin and albendazole were procured from Ind-Swift, Pharmaceutical, Panjab; Savorite Pharmaceuticals, Gujarat; Royal Crop Science, Panipat, India. The melting points of the compounds were determined in open glass capillaries with the help of thermonic melting points apparatus (Campbell Electronics, Mumbai, India) and are uncorrected. The homogeneity of all the newly synthesized compounds were routinely checked by TLC on silica gel G plates and spots were located by using iodine chamber. Elemental analysis was performed in Heraeus CHN rapid analyser. The results were found within the ±0.4% of theoretical values. Infrared spectra were recorded on KBr pellets on a Perkin Elmer system 2000 FTIR spectrometer and 1H- NMR spectra on Bruker DPX 200 using TMS as internal standard.
SYNTHESIS
Synthesis of 2-(1,3,4-thiadiazino (6, 5-b)indole-2´-yl)aminoacetic acid (1)
A mixture of 2-amino-1, 3, 4-thiadiazino (6, 5-b) indole (0.01 mol), chloroacetic acid (0.01 mol) and anhydrous K2CO3 (5.0 gm) in methanol (dry, 80 ml) were refluxed for about 18 h on a water bath. On completion of the reaction, the excess of solvent was distilled off under reduced pressure. At 0-5 °C, 40% hydrochloric acid (0.01 mol) added to residue and a solution of sodium nitrite (0.01 mol) in water (25 ml) was added drop wise during 30 min. The reaction was allowed to stand overnight. Crude reaction mixture was filtered, washed thoroughly with ice cold water and dried in air. The solid thus obtained was recrystallised with ethanol- water to obtain compound 1: Yield 68%, m.p.160 °C. IR (KBr) νmax in cm-1: 1548 (C―C of aromatic), 1568 (N=O), 1615 (C=N), 1710 (C=O of COOH), 2837 (CH2), 3010 (OH of COOH), 3047 (aromatic CH). 1H-NMR (CDCl3) δ: 4.69 (d, 2H, CH2), 6.50-6.80 (m, 4H, ArH), 9.60 (s, 1H, COOH, exchangeable with D2O). MS: m/z 289 [M]+. Elemental analysis (C11H7N5SO3), calcd: C 45.67, H 2.42, N 24.22%, found: C 45.70, H 2.40, N 24.45%.
Synthesis of 2-(Sydnon-3´-yl)-1, 3, 4-thiadiazino (6,5-b)indole (2)
Compound 1 was heated with acetic anhydride (1:5 by weight) on a water bath for 3 h. The reaction mixture was poured over crushed ice and recrystallised with ethanol to get compound 2: Yield 62%, m.p.136 °C. IR (KBr) νmax in cm-1: 841(N-O of sydnones), 1092 (C-O of sydnone), 1252 (C-N), 1522 (N-N), 1573 (C―C of aromatic), 1620 (C=N), 1752 (C=O of sydnone), 3033 (aromatic CH), 3106 (sydnone -CH). 1H-NMR (CDCl3) δ: 4.69 (s, 1H, sydnone), 6.62-6.90 (m, 4H, Ar-H). MS: m/z 271 [M]+. Elemental analysis (C11H5N5SO2), calcd: C 48.70, H 1.84, N 25.83%, found: C 48.76, H 1.86, N 25.80%.
Synthesis of 2-[(4-Sulphoyl chloride)sydon-3-yl]-1,3,4-thiadiazino(6,5-b) indoles (3)
Compound 2 (0.01 mol) taken in small portion to the mixture of chloro sulphonic acid (0.01 mol) and catalytic amount of phosphorous pentoxide. Shake well to ensure thorough mixing. The resulting mixture heated at 60-65 0C for about 1 hr. On completion the reaction, reaction mixture allowed to cool and pour the mixture over ice. Stirred, filtered, washed and dried to get compound 3. Yield 69%, m.p.194 °C. IR (KBr) νmax in cm-1: 845(N-O of sydnones), 1090 (C-O of sydnone), 1180 (SO2), 1247 (C-N), 1526 (N-N), 1570 (C―C of aromatic), 1625 (C=N), 1749 (C=O of sydnone), 3030 (aromatic CH). 1H-NMR (CDCl3) δ: 6.57-6.95 (m, 4H, Ar-H). MS: m/z 369.5 [M]+. Elemental analysis (C11H4N5S2O4Cl), calcd: C 35.72, H 1.08, N 18.94%, found: C 35.61, H 1.09, N 18.80%.
General preparation of 2-[{(4-Substituted-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino (6,5-b)indoles (4a-f)
A mixture of dimethyl formamide and various 2º-amine (0.02 mol) was added to compound 3 (0.02 mol) with stirring for 2 hr. Catalytic amount of pyridine was added to reaction mixture. The reaction mixture was poured to the ice cold water. Crude products were recrystallized in appropriate solvents to furnish compounds 4a-f.
4a: Yield 69%, m.p.194 °C. IR (KBr) νmax in cm-1: 845 (N-O of sydnones), 1090 (C-O of sydnone), 1180 (SO2), 1247 (C-N), 1526 (N-N), 1570 (C―C of aromatic), 1625 (C=N), 1749 (C=O of sydnone), 3030 (aromatic CH). 1H-NMR (CDCl3) δ: 2.02-2.51 (t, 8H, -N-CH2), 6.52-7.00 (m, 4H, Ar-H). MS: m/z 420.10 [M]+. Elemental analysis (C15H12N6S2O5), calcd: C 42.86, H 2.86, N 20.00%, found: C 42.97, H 2.82, N 19.97%.
4b: Yield 51%, m.p.132 °C. IR (KBr) νmax in cm-1: 851 (N-O of sydnones), 1097 (C-O of sydnone), 1182 (SO2), 1253 (C-N), 1530 (N-N), 1576 (C―C of aromatic), 1631 (C=N), 1742 (C=O of sydnone), 3031 (aromatic CH). 1H-NMR (CDCl3) δ: 1.83 (s, 1H, NH), 2.10-2.62 (t, 8H, -N-CH2), 6.60-7.12 (m, 4H, Ar-H). MS: m/z 419.10 [M]+. Elemental analysis (C15H13N7S2O4), calcd: C 42.95, H 3.10, N 23.38%, found: C 42.91, H 3.12, N 23.46%.
4c: Yield 48%, m.p.159 °C. IR (KBr) νmax in cm-1: 849 (N-O of sydnones), 1094 (C-O of sydnone), 1184 (SO2), 1250 (C-N), 1533 (N-N), 1573 (C―C of aromatic), 1628 (C=N), 1747 (C=O of sydnone), 3028 (aromatic CH). 1H-NMR (CDCl3) δ: 2.06-2.69 (t, 10H, -N-CH2), 6.60-7.12 (m, 4H, Ar-H). MS: m/z 418.23 [M]+. Elemental analysis (C16H14N6S2O4), calcd: C 45.91, H 3.35, N 20.10%, found: C 45.88, H 3.36, N 20.15%.
4d: Yield 57%, m.p.168 °C. IR (KBr) νmax in cm-1: 854 (N-O of sydnones), 1092 (C-O of sydnone), 1180 (SO2), 1251 (C-N), 1536 (N-N), 1572 (C―C of aromatic), 1637 (C=N), 1740 (C=O of sydnone), 3033 (aromatic CH). 1H-NMR (CDCl3) δ: 1.22 (s, 3H, N-CH3), 2.15-2.66 (t, 8H, -N-CH2), 6.60-7.12 (m, 4H, Ar-H). MS: m/z 433.41 [M]+. Elemental analysis (C16H15N7S2O4), calcd: C 44.30, H 3.46, N 22.61%, found: C 44.27, H 3.40, N 22.69%.
4e: Yield 52%, m.p.147 °C. IR (KBr) νmax in cm-1: 848 (N-O of sydnones), 1095 (C-O of sydnone), 1178 (SO2), 1250 (C-N), 1530 (N-N), 1574 (C―C of aromatic), 1630 (C=N), 1744 (C=O of sydnone), 3035 (aromatic CH). 1H-NMR (CDCl3) δ: 2.10-2.60 (t, 8H, -N-CH2), 6.79-7.52 (m, 9H, Ar-H). MS: m/z 495.12 [M]+. Elemental analysis (C21H17N7S2O4), calcd: C 50.89, H 3.43, N 19.79%, found: C 50.87, H 3.44, N 20.02%.
4f: Yield 60%, m.p.182 °C. IR (KBr) νmax in cm-1: 853 (N-O of sydnones), 1090 (C-O of sydnone), 1185 (SO2), 1255 (C-N), 1533 (N-N), 1570 (C―C of aromatic), 1636 (C=N), 1743 (C=O of sydnone), 3030 (aromatic CH). 1H-NMR (CDCl3) δ: 1.34 (t, J = 6 Hz, 3H, -CH3), 2.78 (q, J = 4 Hz, 2H, -CH2-), 2.10-2.60 (t, 8H, -N-CH2), 6.60-7.12 (m, 4H, Ar-H). MS: m/z 447.18 [M]+. Elemental analysis (C17H17N7S2O4), calcd: C 45.61, H 3.80, N 21.91%, found: C 45.67, H 3.75, N 22.00%.
CONCLUSION
Pharmacological evaluation data revealed that incorporation of secondary amines into compound 3 i.e. 2-[(4-Sulphoyl chloride)sydon-3-yl]-1,3,4-thiadiazino(6,5-b) indoles brought significant efficacy in to biological activity. Compound 4a & 4e were the only compounds which displayed significant antimicrobial activity against all the selected panel of pathogens but order of efficacy was 4a > 4e. During insecticidal activity against P. americana, compound 4a showed remarkable potential in comparison to used standard cypermethrin. Again compound 4a was the only compound who showed better antihelmitic activity in compare to applied albendazole standard. On the basis of SAR, morphloine substitution seems to be very vital for biological activity profile. Morpholino sydnonyl substituted indole i.e. 2-[{(4-morpholino-1-sulphonyl)sydnon-3-yl}]-1,3,4-thiadiazino(6,5-b)indole 4a, showed promising drug canditure among the all screened derivatives.
Acknowledgements
We are thankful for SAIF, Punjab University, India for spectral, elemental analysis and Department of Pathology, L.L.R.M. Medical College, India for biological activities.
REFERENCES
- Clapp L. B. and Katritzky A. R., In Comprehensive Heterocyclic Chemistry; Pergamon: Oxford, 4B(6), 365-391 (1984) (b) Browne, D. L.; Harrity, J. P. A. Tetrahedron, 66, 553-568 (2010).
- Newton C.G. and Ramsden C. A., Tetrahedron, 38: 2965-3011 (1982).
- Stewart F.H.C., Chem. Rev., 64: 129-147 (1964).
- Kamble R.R. and Sudha B.S., Indian J. Pharm. Sci., 68(2): 249-253(2006).
- Butkovic K., Marinic Z. and Sindler-Kulyl M., Arkivoc , 10: 1-15 (2011).
- Kier B.L., Dhawan D. and Fregly J.M., J. Pharm. Sci., 53: 677-678 (1964).
- Jogul J.J. and Badami B.V., J. Serb. Chem. Soc., 71: 851-860 (2006).
- Shahrukh T.A., Nikul S.P. and Keshav C.P., Org. Commun., 3: 30-38 (2010).
- Kier L.B., Al-Shamma A., Hann R. and Tye A., J. Pharm. Sci., 55: 1467-1468 (1966).
- Satyanarayana K., Deshpande R.S. and Subbarao B., Indian J. Pharm. Sci., 66(5): 679-683 (2004).
- Satyanarayana K. and Rao M.N.A., Ind. J. Pharm. Sci., 57(6): 243-248 (1995).
- Latthe P. R., Shinge P. S., Bharati V. B., Patil B. P. and Holihosur S. N., J. Chem. Sci., 118(3): 249 (2006).
- Rahiman M. A. and Kalluraya B., J. Ind. Council Chem., 25(1): 10-14 (2008).
- Rani P., Srivastava V.K. and Kumar A. Eur. J. Med. Chem., 39: 449-452 (2004).
- Andreani A., Rambaldi M., Locatelli A., Conti M. and Malandrino S., Acta Pharm. Nord., 3(1): 5-8 (1991).
- Chavan R.S., More H.N. and Bhosale A.V., Int. J. Pharm. Biomed. Res., 1(4): 135-143 (2010).
- Bansal E., Srivastava K.V. and Kumar A., Indian J. Chem., 39B: 357-362 (2000).
- Amir M., Dhar N. and Tiwari K.S., Indian J. Chem., 36B: 96-98 (1997).
- Stanton J.L. and Ackerman M.H., J. Med. Chem., 26(7): 986-999 (1983).
- Zahran H.A.M. and Ibrahim M.A., J. Chem. Sci., 121(4): 455-462 (2009).
- Sharma P., Kumar A. and Pandey P., Indian J. Chem., 45B: 2077-2082 (2006).
- Biradar J.S. and Manjunath S.Y., Indian J. Chem., 43B: 389-392 (2004).
- Pardasani R.T., Pardasani P., Sherry D. and Chaturvedi V., Indian J. Chem., 40B: 1275-1278 (2001).
- Ryu C.K., Lee J.Y., Park R.E., Ma M.Y. and Nho J.H., Bioorg. Med. Chem. Lett., 17(1): 127-131 (2007).
- Devi R. and Biradar J.S., Ind. J. Chem., 39B: 929-935 (2000).
- Panwar H., Chaudhary N., Singh S. and Chawla A., Kor. J. Chem., 55(6): 994-999 (2011).
- Panwar H., Verma S.R. and Srivastava K.V., Indian J. Chem., 45B: 2099-2104 (2006).
- Koch С W, Milberg R. M. and Markgraft J. H., J. Heterocycl. Chem., 10: 973-978 (1973) (b) Charles W. K., Richard M. M., Robert J. K., Markgraf J. H. and Wege P. M., J. Heterocycl. Chem., 11: 475-479 (1974).
- Bowie J.H., Eade R. A. and Earl J. C., Aust. J. Chem., 21(6): 1665-1670 (1969) (b) Gouldie R. S., Preston P. N. and Palmer M. H., Org. Mass spectrum., 2(10): 953-963 (1969) (c) Ollis W. D. and Ramsden C. A., J. Chem. Soc. Perkin Trans., 1: 645-650 (1974).
- Cruickshank R., Duguid J. P., Marion B. P. and Swain R.H., In Medicinal Microbiology,12: Churchill Livingstone: London, U.K. (1975).
- Collins H.A., Microbiological Methods, Butterworth, London, U.K., 2: (1976).
- Khan K.Z., In vitro and vivo screening techniques for bioactivity screening and evaluation, in Proceedings of the International Workshop on UNIDO-CDRI (1997).
- Varma S.R., Antifungal Agents:Past, Present and Future Prospects, National Academy of Chemistry and Biology, Lucknow, India (1998).
- Nirmal S. A., Malwadkar G. and Laware R. B., Songklanakarin J. Sci. Technol., 29(3): 755-757 (2007).
- Tambe V.D., Nirmal S.A., Jadhav R.S., Ghogare P.B., Bhalke R.D., Girme A.S. and Bhamber R.S., Ind. J. Nat. Prod., 22: 27-29 (2006).
- Thorn G.W., Adams R.D. and Petersdrof R. G., Harrison’s Principles of Internal Medicine (McGraw Hill Co, New Zoology Department, Prof. M. Bhide, Department of York), 1088-1089 (1977).
- Vilgar Z., Atlas of Medical Parasitology (PG Publishing kind assistance for performing antimicrobial studies House, Singapore), 216-217 (1984).

This work is licensed under a Creative Commons Attribution 4.0 International License.









