Introduction
Polymeric nanofibers are a prime subclass of nanomaterials. The nanofibers have a diameter between 0.01 and 1 micrometer, in other words, they can range from micrometer to sub-micrometer levels.1 They have excellent properties such as mimicable surface functionalities, good mechanical properties for example excellent stiffness and tensile strength and very high surface area to volume ratio.2, 3
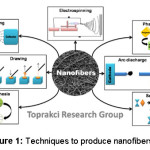
As shown in figure 1, there are several techniques to produce nanofibers. Techniques such as melt-spinning,1,4 electrospinning,1,2,5 phase separation,1, 6, 7 template synthesis1,8,9 and self-assembly1,10 can be used to produce nanofibers. Among these methods, the most commonly used one is electrospinning method. This method is continuous, economical and easy.1
Figure 1: Techniques to Produce Nanofibers.
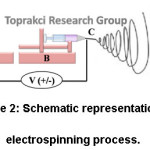
Electrospinning process has four fundamental components (Fig. 2). The first is a high voltage power supply (Fig. 2A) that gives power to get the charged polymer solution into a fiber form. The latter is a syringe pump (Fig. 2B) to control the flow rate of the polymer solution. The third component is the needle to disperse charge on polymer jet (Fig. 2C). The last one is the collector (Fig. 2D) that picks up electrospun fibers by discharging them.11, 12
Figure 2: Schematic Representation of Electrospinning Process.
Polymers used in nanofiber production are of importance due to mechanical, electrical and thermal properties [13]. Ideal polymers for biomedical applications should be both non-toxic, biodegradable, biocompatible and mechanically strong. Nanofibers can be manufactured from polymer/polymer blend melt or solution. Poly (lactic acid) (PLA), poly (vinyl alcohol) and polycaprolactone are examples of biocompatible polymers. Due to its biodegradability, biocompatibility and spinnability, PLA is a widely used polymer in electrospinning processes in versatile biomedical areas.14,15 PLA can be easily dissolved in different kinds of conventional solvents such as acetone (AC), chloroform (CHL), dichloromethane (DCM), dimethylacetamide (DMAc), dimethylformamide (DMF), 1;4-dioxane (DX), tetrahydrofuran (THF).16-19
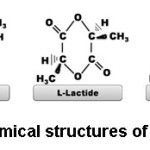
PLA can be produced by condensation polymerization and ring opening polymerization techniques. PLA obtained by condensation polymerization has low molecular weight and poor mechanical properties. Physical properties of PLA have shown excellent improvement with ring opening polymerization.20 In this method, PLA is synthesized by using lactide monomer. Lactide is the cyclic dimer of lactic acid. As shown in figure 3, lactide can be found in different chemical forms such as D-lactide, L-lactide and meso-lactide (L:D-lactide).
Figure 3: Chemical Structures of Lactide Types.
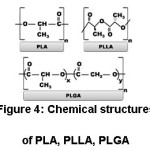
Poly-L-lactic acid (PLLA) occurs with polymerization of L-lactide.21 Poly (lactide-co-glycolide) (PLGA) is one of the most commonly used copolymers of PLA in biomedical applications. PLGA occurs by copolymerization of PLA and polyglycolic acid (PGA).22 Chemical structures of PLA, PLLA and PLGA are shown in the Figure 4.
Figure 4: Chemical Sstructures of PLA, PLLA, PLGA
Nanofibers of PLA and PLA blends can be used in a wide variety of biomedical and biotechnological applications (Table 1). Biomedical applications include drug release, scaffold for tissue engineering, dressings for wound healing and dental applications while biotechnological applications contain biosensors, molecular filtrations and preservations of biological agents.1
This review focuses on electrospun nanofibers of PLA and PLA blends used in biomedical applications in recent years. Advantages and limitations of electrospinning technique for biomedical applications are described in the review. In this study, biomedical applications are categorized as wound dressings, dental applications, controlled drug release and tissue engineering. Future perspectives related with electrospun PLA based nanofibers for biomedical applications were also discussed.
Biomedical Applications
The use of polymeric nanofibers for biomedical applications has some internal advantages such as deposition in fibrous forms or constructions and mimicking or replicating bio-systems. Wound dressings, dental applications, controlled drug release and tissue engineering are described in detail below within the context of biomedical applications.
Table 1: Details of Various Electrospun PLA Based Nanofibers for Biomedical Applications
|
Polymers and Blends
|
Molecular weight (g/mol)
|
Solvents
|
Average fiber diameter (nm)
|
Fillers
|
Application
|
Reference
|
|
PLA
|
200,000
|
DMAc and
CHL
|
971 ± 274
|
Cur
|
Wound healing
|
[23]
|
|
PLLA and PVDF
|
90,000
|
DMAc and
AC
|
2,290 – 4,620
|
Cur and Enro
|
Wound healing
|
[24]
|
|
PLGA
|
120,000-
190,000
|
Trifluoroethanol and CHL
|
777 ± 249
|
Ciprofloxacin and sodium alginate
|
Wound healing
|
[25]
|
|
PLLA
|
300,000
|
DCM
|
3000-5000
|
DMOG and DS
|
Wound healing
|
[26]
|
|
PLGA
|
75,000
|
1,1,1,3,3,3- hexafluoro-2-propanol (HFIP)
|
~1,000
|
–
|
Cardiac
|
[27]
|
|
PLGA
|
110,000
|
HFIP
|
720 ± 350
|
Elastin and collagen
|
Cardiac
|
[28]
|
|
PLA
|
75,000-
120,000
|
DMF, DCM and methanol
|
~556
|
Dipyridamole
|
Cardiac
|
[29]
|
|
PLLA
|
100,000
|
HFIP
|
100–500
|
Laminin
|
Nerve
|
[30]
|
|
PLLA
|
100,000
|
HFIP and hexamethyl-disilazane (HMDS)
|
860 ± 110
|
Coll and HA
|
Bone
|
[31]
|
|
(PPDO:PLLA:- PEG)
|
42,000
|
DMF and DMC
|
~1,400
|
–
|
Skin
|
[32]
|
|
PLA and PEVA
|
205,000
|
CHL and methanol
|
1,000–3,000
|
Tetracycline
|
Drug release
|
[33]
|
|
PLLA
|
–
|
CHL, 1,2-dichlorethane and ethyl acetate
|
290 – 539
|
Cyclosporine A (CsA)
|
Drug release
|
[34]
|
|
PLA
|
470,000
|
DMF and DCM
|
549.7 ± 153.1
|
Graphene oxide (GO) and rhodamine B
|
Drug release
|
[35]
|
|
PLLA and chitosan
|
low molecular weight
|
AC and DCM
|
2 760 ± 720
|
–
|
Dental
|
[36]
|
Dressings for Wound Healing
There is an increasing demand for wound care products due to various types of wounds such as traumatic wounds, acute wounds and chronic wounds. With this demand, the development of dressing materials for wound healing is getting more importance.1 An ideal dressing should provide an environment on the surface of the wound where the healing can occur at maximum rate consistent with wound healing and an acceptable cosmetic appearance.1,37
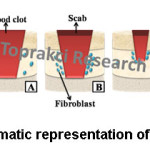
The wound healing occurs in 4 fundamental phases (Fig. 5). First phase is hemostasis phase (Fig. 5A). The aim of this phase starting with the beginning of injury is to stop the bleeding. Second phase is called inflammatory phase (Fig. 5B). This phase destroys bacteria and prepares the wound bed for the growth of new tissue. Third phase is proliferation phase (Fig. 5C). Filling the wound, contraction of the wound margins and covering the wound is in the proliferative phase. Maturation phase (Fig. 5D) is last phase. The new tissue acquires flexibility and strength in the maturation phase.38
Figure 5: Schematic Representation of Wound Healing
There are some important parameters in nanofiber mats for wound healing such as fiber diameter, tensile strength and surface properties (i.e., hydrophilicity or hydrophobicity). Relative humidity affects directly wound healing process. Relatively humid conditions can create good environment for bacterial growth, propagation and further infection. This phenomenon adversely affects wound healing process. So, good wound dressing material should have low stiffness, high flexibility, good mechanical properties and bifunctional surface character (i.e., wound contacting surface to wound should have hydrophilic character to absorb blood and wound discharge from scar tissue, on the other hand, environmental contacting surface of wound dressing material should have hydrophobic surface to prevent moisture penetration into wound area). While PLA, PLLA and PLGA are relatively hydrophobic polymers compared with PVA and natural polymers, on the other hand they can show relatively higher hydrophilicity compared with polyethylene, polypropylene, PVdF etc.
Nguyen et al., has produced curcumin-loaded PLA nanofiber mats to develop a functional wound dressing.23 PLA nanofibers had random fiber orientation and 971 ± 274 nm average diameter. The average diameter and distribution of the nanofiber diameters were decreased with increasing curcumin (Cur) amount. The tensile stress of PLA nanofibers was 2.5 MPa and it increased to level of 3.5 MPa with an increase in Cur levels from 0.125% to 1.25%. A further increase in Cur levels up to 6.25 wt% resulted in a decrease in tensile strength. Obtained mechanical properties were satisfactory for wound dressing materials. In vivo wound healing experiments were investigated using PLA and cur-loaded PLLA nanofiber treatments in a mouse model. In their study, PLA is preferred due to its biodegradability, biocompatibility and its ability to support the multiplication and binding of various cells. It was observed that tissue interaction with Cur increased because of the high porosity and large surface area.23
A sandwich-structured composite (SSC) was manufactured by Jinzhen Li et al., Layers were PVdF and enrofloxacin (Enro)/PLA while interface were Cur/PLA. Cur/PLA layer had diameters ranging from 2.29 to 4.62 µm and random fiber orientation. The tensile strength of SSC membranes was higher than 3.1 MPa. It was observed that SSC membranes showed both hydrophilic and hydrophobic surface character. The hydrophilic surface (Enro/PLA) was touched to the wound to release drugs for the wound healing. The hydrophobic surface (PVdF) was prevented penetration of moisture and also reduced the wound infection chance. Enro and Cur were distributed homogeneously in the SSC membrane matrix according to their results. These membranes had good biocompatibility, flexibility, mechanical strength and antibacterial effect against well-known bacterial cultures such as E. coli, S. pneumoniae, P. aeruginosa, S. aureus and C. albicans.24
Ciprofloxacin (antibiotic)-loaded electrospun PLGA mats blended with sodium alginate were prepared by Liu et al., PLGA nanofibers had random fiber orientation and 777 ± 249 nm average diameter. The diameter of PLGA fibers were considerably decreased with the addition of sodium alginate. Addition of ciprofloxacin was not significantly affected the fiber diameters in comparison with diameters of PLGA/sodium alginate fibers. Wetting capability of sodium alginate was increased capacity of water absorption according to their results. Water uptake was increased with addition of sodium alginate. The stiffness of PLGA mat was decreased with adding sodium alginate, so the injured area was protected much better. The tensile strengths of produced mats were higher than 3 MPa. The burst release of ciprofloxacin was supplied advanced antibacterial effect to the PLGA mats with adding sodium alginate.25
Low formation of blood vessels (angiogenesis) and late wound closure are seen in diabetic patients. PLLA electrospun membranes including dimethyloxalylglycine (DMOG)-loaded mesoporous silica nanoparticles (DS) was fabricated for diabetic wound healing by Ren et al., PLLA electrospun mats had aligned fibers with around 3000 -5000 nm diameter and elliptic-shaped pores on the surface of fibers. Also, the diabetic mice were used for analysis of wound healing efficiency in their study. Produced nanofibers were placed in the dorsal area of mice. The wound area was decreased with time. In the end of 15 days, around 94% healing ratios had been seen.26
Nanofibrous Scaffolds for Tissue Engineering
Biodegradable scaffolds can be seen as an important class for tissue engineering. They are utilized as temporary templates prior to renewing of natural extracellular matrix or biologically functional tissue. The similarity of the fibrous scaffold with native tissue is significant which allows the growth of the artificial texture. The fibrous scaffolds should allow copying the physical structure of the natural extracellular matrix for regeneration of tissue.1,39,40
The usage areas of nanofibers are determined according to their physical and chemical properties such as porosity, biodegradability and mechanical properties. Nanofibers can be used in many tissue engineering applications such as cardiac,41-44 nerve,45-49 bone50-54 and skin.55-59 These areas are described in detail within the following sections.
Nanofibers for Cardiac Tissue Engineering
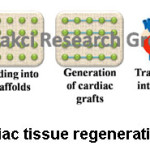
Heart diseases are one of the main causes of death. 366,800 people are died a year due to coronary heart disease according to American Heart Association statistics in 2018. It means that 1 in 7 deaths in the US are because of coronary heart disease. According to World Health Organization statistics, ischemic heart disease remains the first cause of death in the world.60,61 Cardiac tissue engineering presents new solutions for patients with heart failure. 12, 62-64 As shown in figure 6, firstly scaffold for cardiac tissue should be prepared. Then, seeding of repairing cells into scaffold should be carried out in order to generate cardiac grafts. In the final step, cardiac tissue construct should be transplanted onto heart for heart regeneration.
Figure 6: Cardiac Tissue Regeneration Mechanism
Zong et al., worked with electrospun PLGA-based scaffolds to produce of cardiac tissue constructs. Average diameter of fibers was 1 µm. After fibers were produced as random, they were uniaxially stretched. Scaffolds were investigated for the attachment and growth of the cardiac myocytes (CMs). Hydrophobic surfaces were comparatively preferred by CMs. When the amount of GA in PLGA was increased, degradation rate was increased and pH was decreased. CMs were badly affected because of faster degradation rate. Optical mapping of electrical activity was prepared in cultured CMs. Shorter action potential durations and external pacing rates up to 6 Hz were exhibited by cultured CMs on PLLA membranes. ,according to their results, electrospun PLGA scaffolds were ensured guidance and flexibility to grow CMs. PLGA scaffolds were used favorably to get cardiac tissue.27
In another study, vascular scaffolds were fabricated as a native vessel by Stitzel et al., Elastin, collagen and PLGA were used with different ratios for this study. Produced scaffolds were designed to be 1 mm thick and 12 cm long. In their study, fibers had diameters of 0.72 ± 0.35 µm and a random orientation. Mechanical properties of vascular scaffolds were improved by adding PLGA. While the diameter change of the natural vessel was 9%, the diameter change of the electrospun scaffolds was 12-14%. This was the evidence of similar behavior between the natural vessels and the electrospun scaffolds. According to their results, produced scaffolds were resisted at nearly 12 times higher pressure than systolic pressure – taken during each beat of the heart. Endothelial and smooth muscle cells were used for biocompatibility test. An average of 82% of smooth muscle cells and 72% of endothelial cells were stayed alive on the scaffold according to mitochondrial metabolic activity assay. An average of 80% of smooth muscle cells and the endothelial cells were stayed alive on the scaffold according to neutral red assay. The biocompatibility of the produced scaffolds were demonstrated using in vivo mice models. This study was showed the promise of a functional vascular graft in clinical applications.28
Drug-eluting cardiovascular stents have been developed to avoid blood clot in recent years. Dipyridamole is a well-known medication and inhibits blood clot formation. Drug-eluting cardiovascular stents were fabricated by Bakola et al., In their study, dipyridamole loaded PLA nanofibers were prepared. Produced PLA fibers had 522 nm average fiber diameter and a random orientation. Hydrophobic behavior was exhibited by PLA and dipyridamole loaded PLA scaffold according to contact angle measurements. Contact angle of PLA and dipyridamole loaded PLA scaffolds were observed at 124.9° ± 0.4 and 129.3° ± 1.7, respectively. While roughness and surface wetting properties of scaffold were decreased with the addition of the drug, contact angle values and scaffold’s hydrophobicity were increased. Dipyridamole loaded PLA scaffold was placed upon cardiovascular Co/Ni stent. Well scaffold morphology and enough adhesion upon the stent was observed.29
Nanofibers for Nerve Tissue Engineering
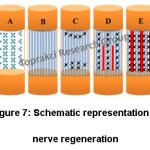
Regeneration within a hollow nerve guidance conduit (NGC) is observed to understand nerve regeneration. As shown in figure 7, regeneration process is comprised of 5 main phases. These phases are the fluid phase, the matrix phase, the cellular migration phase, the axonal phase and the myelination phase.65,66 First phase is nerve conduit repair. An influx of plasma exudate is released from both the proximal and distal nerve stumps in the initial fluid phase (Fig. 7A).65-68 Second phase is formation of fibrin cable between the proximal and distal stumps (Fig. 7B). Cell migration, proliferation and alignment and tissue cable formation is occurred in the third phase (Fig. 7C).65-67 Growth of axons should be seen in the fourth phase (Fig. 7D). Axonal fibers are used as guidance in the myelination phase (Fig. 7E).66 Developing neural guidance conduits and directing the extension of nerve cells are the main objectives of nerve tissue engineering. One of the important factors of tissue engineering is bioactive molecules. Especially in nerve tissues, neurogenesis is increased by bioactive molecules. These molecules are expensive, unstable and also have also side effects, generally.69 According to literature, electrospun nanofibers with different morphologies and structures (oriented, hollow, core-sheath, sponge-like threads etc.) are suitable for nerve regeneration.12,70 Electrospun nanofibers loaded with active molecules such as laminin,30 melanin71 gelatin,45 fibrinogen from bovine plasma72 and lycium barbarum polysaccharide69 are good candidates to fulfill scaffold requirements for nerve regeneration.
Figure 7: Schematic Representation of Nerve Regeneration
Koh et al., studied the laminin loaded PLLA nanofibers for nerve regeneration applications. Three different methods were used in their study. After PLLA nanofibers were produced by electrospinning, plasma-treatment and chemical bonding of laminin onto PLLA nanofibers was used in the first method. In the second method, after electrospinning PLLA nanofibers, plasma-treatment and physical adsorption of laminin onto PLLA nanofibers was made. In the last method, only electrospinning method was performed with blended laminin-polymer solution to obtain laminin loaded PLLA nanofibers. PLLA and functionalized laminin–PLLA random nanofibers were consisted of nanofibers in the diameter range of 100–500 nm. Uniform distribution of laminin was observed on the nanofibers according to morphological characterization. According to their results, blended laminin–PLLA nanofibers had more laminin comparing to others and also showed better neurite growth rate compared with other chemical and physical bonding routes.30
Nanofibers for Bone Tissue Engineering
Bone is a strong, rigid and hard binding tissue by contrast with soft tissue. Natural bone is an organic-inorganic biocomposite. It consists of 30% of organic matrix and 70% inorganic crystals by weight. Bone grafts are good choices after bone fracture, loss and infection. They must ensure suitable environment for osteogenesis.12,55 Bone formation can occur in two ways: intramembranous and endochondral bone formation. Intramembranous bone formation includes bone tissue formation from embryonic connective tissue. In endochondral bone formation, hyaline cartilage occurs as outline of the future bone. Cartilage tissue is damaged in this formation and formed the basis for bone tissue.73 Bone reparation contains repeated bone formations, so there should be no scar on bone at the end of healing period. Intramembranous bone formation starts at cortex and periosteum. The fracture is stabilized by external soft tissues, and then cartilage occurs. While tissues grow mature and the matrix calcifies, formation of cartilage decreases. Chondroclasts are carried by growing blood vessels, then new bone formation start. While new bone reshape, mechanical continuity is provided.74
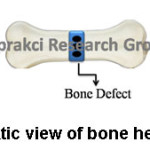
As shown in figure 8, bone healing mechanism occurs in 3 steps. Each step is related with the mechanical aspects. First step involves the bone mechanical properties and loading conditions. Scaffold should not contain a stress-shielding effect at this step. So, the scaffold’s elastic modulus should not be higher than that of the main bone. Interface biomechanics is the second step. The scaffold mechanical properties adjust to manufacture scaffold-bone mechanotransduction at the second step. This mechanotransduction affects the differentiation of tissue.75 Third step known as final fixation includes degradation of scaffold. When scaffold degrades, the mechanical load is supported by ingrown bone.76
Figure 8: Schematic View of Bone Healing Mechanism
Electrospun nanofibers loaded with active bone healers such as hydroxyapatite (HA),31,77-80 β-tricalcium phosphate (β-TCP),81 collagen (coll),51,82 siloxane83 and zinc-cur84 are good candidates to fulfill scaffold requirements for aforementioned bone healing mechanism.
PLLA, PLLA/hydroxyapatite(HA) and PLLA/coll/HA scaffolds were fabricated by Prabhakaran et al. Fiber diameters of the electrospun PLLA, PLLA/HA and PLLA/coll/HA nanofibers were 860 ± 110, 845 ± 140, 310 ± 125 nm, respectively. Produced nanofibers had a random orientation and bead free. While average fiber diameters of PLLA and PLLA/HA were similar, fiber diameters were decreased considerably with collagen addition. The average tensile strength of PLLA, PLLA/HA and PLLA/coll/HA scaffolds were 4.69 ± 0.19, 3.10 ± 0.15, 2.05 ± 0.10 MPa, respectively. Elongation at break was also similar for PLLA (25%) and PLLA/coll/HA (28.95%) scaffolds. In their study, proliferation of human fetal osteoblasts (hFob) and mineralization process were investigated. Proliferation on PLLA/HA scaffold was better than proliferation on PLLA scaffold. Among their samples, the best proliferation was seen in PLLA/coll/HA scaffolds. According to their morphological results, the best mineralization was also seen in PLLA/coll/HA scaffolds.31
Nanofibers for Skin Tissue Engineering
Nanofiber scaffolds preserve the wound field from decrement of fluid and proteins and bring better appearance. Fibers support cell adhesion and proliferation. Therefore, nanofiber scaffolds show promise for skin tissue engineering.55,57 Electrospun nanofibers loaded with active materials such as silk fibroin,85 bioactive glass,86 gelatin 87,88 and poloxamer58 are good candidates to fulfill scaffold requirements.
Poly(p-dioxanoneco-L-lactide) block-poly(ethylene glycol) scaffolds were produced by Bhattaraiet al. Copolymer were consisted of random nanofibers in the diameter of 1.4 µm. This product had >80% porosity and 1.4 MPa tensile strength. Proliferation, fibroblast adhesion and cell growth along the fiber were promoted by the scaffolds.32
Drug Release
Release of drugs or pharmaceutical agents in desired ratio is very important for patients.1 A drug release system is designed to check drug delivery in desired ratio and time.12,89 Drug release with polymer nanofibers, one of the new technologies, has some advantages due to their high aspect ratio. The rate of drug release can be determined by nanofiber characteristics such as fiber diameter, porosity and fiber/drug binding properties.12,90 The structure of the polymer and the state of the drug are very important at the nanofiber-based drug delivery systems. A fast burst release and then a slower sustained release are occurred at the drug release from semi-crystalline polymer nanofibers.33 The interaction between drug and the polymer also affects nanofiber-based drug delivery system.91-93
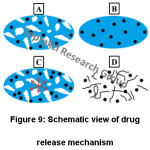
There are three main mechanisms for a controlled drug delivery system. These are diffusion, chemical reaction and solvent activation. Diffusion-controlled system is the most common drug delivery system.93,94 The rate of drug diffusion is affected by different factors such as molecular geometry, drug-matrix interactions, etc. Encapsulated drug is a large hydrophilic molecule, so it cannot diffuse through the polymer. Thus, polymer swells with osmotic pressure. It is called true release mechanism. If the release mechanism is described as the drug release way, a 4-step release process occurs in that system. As shown in figure 9, these are diffusion through water-filled pores (Fig. 9A), diffusion through the polymer (Fig. 9B), osmotic pumping (Fig. 9C) and erosion (Fig. 9D).95 Various drugs such as tetracycline,33 cyclosporine A (CsA),34 Rhodamine B (RhB),35 dipyridamole,29 ketoprofen (KET)96-98 are used in drug release.
The study of Kenawy et al., is the first report for drug release from electrospun polymers. Electrospun mats of PLA, poly (ethylene-co-vinylacetate) (PEVA) and PLA/PEVA blend were produced in their study. PLA/PEVA nanofibers were consisted of random nanofibers in the diameter range of 1–3 µm. Tetracycline release of mats was observed in their study. The drug release was relatively smooth during 5 days according to their results. This work demonstrates that electrospun polymer matrixes can be used in controlled release technology.33
Figure 9: Schematic View of Drug Release Mechanism
Another study, Holan et al., worked with dissolved immunosuppressive drug of cyclosporine A (CsA) in PLLA solution. The addition of CsA was not affected structure of the nanofibers and parameters of the electrospinning. Produced nanofibers were consisted of random nanofibers in the diameter range of 290 to 539 nm and contained 1 μg of CsA mm-2. The pharmacological activity of CsA was not changed during the process. The CsA in culture environment was released during at least 96 hours. At the same time,35 percent of the drug was still conserved inside the nanofibers on day 8. CsA-loaded nanofibers were added inside mouse spleen cells. According to their results, PLLA nanofibers can be used as drug carriers and scaffolds.34
In another study, electrospun PLA/graphene oxide (GO) nanofiber membranes for drug release were produced by Mao et al., Produced nanofibers as co-axial structure were consisted of random nanofibers in the diameter range 549.7 ± 153.1 nm for co-axial structure with 25 mg mL-1 of GO. Rhodamine B (RhB) was used as a drug model. The release of RhB from the nanofibers was increased with addition of GO. According to the results, PLA/GO nanofiber membranes were good option for drug release systems.35
Drug-eluting cardiovascular stents were fabricated by Bakola et al., as described in the cardiac tissue engineering section, previously. Drug-release of the dipyridamole loaded PLA scaffold was discussed within this section. The pharmacokinetics of dipyridamole loaded PLA scaffolds was observed for nearly 7 months. 13% dipyridamole was released at first burst after the first 24 hours. Slow and controlled release was observed subsequently. 82% dipyridamole was released in 182 days. 100% of the drug was released in 218 days at last. According to their results, extreme drug release was observed in the first 24 hours. While the polymeric matrix disintegrated, constant and controlled drug release was observed. Lastly, drug release was slow and low.29
Dental Applications
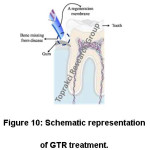
Periodontal diseases are the common cause of tooth loss. Connective tissue weakens and bone support is lost with this disease.36,99 There are some traditional treatment methods like stem cell therapy,100 guided tissue regeneration (GTR)36 and bone graft101 for periodontal diseases. GTR is the most promising traditional treatment methods.36, 102-106 GTR treatment (Fig. 10) involves implanting a regeneration membrane to restore the tissue.36,102 Regeneration membrane must be biocompatible to complete tissue regeneration.36
Figure 10: Schematic Representation of GTR Treatment.
Nonresorbable materials such as expanded polytetrafluoroethylene (e-PTFE) were used in the first GTR treatments.36,105 However, these materials make it difficult to make the necessary stitches and require a second surgical procedure to remove these materials.36,107 Before regeneration of tissues and bone defects, a regeneration membrane should be degraded quickly. For this reason, nature-derived materials, i.e. PLA, are good candidates due to their controllable degradation time and biocompatibility.36,108
PLLA has been used in dental surgery due to its easy processability and biodegradability.36,109 Trejo et al., implanted PLLA barriers of 30 patients for intrabony defects. The cure was showed result well, but applications are limited because of hydrophobic character of PLLA. Difficulties are encountered in fixing the cells to the bones.110
Chen et al., fabricated successfully PLLA/chitosan membrane for the regeneration of periodontal guided tissues. After PLLA was produced with electrospinning method, chitosan was grafted on PLLA fibers. PLLA-CS nanofibers were consisted of random nanofibers in the diameter range of 2.76 ± 0.72 µm. Hydrophilicity, degradation and the biocompatibility was increased with chitosan according to their results.36
Conclusion
Electrospun PLA-based nanofibers show great potential in biomedical applications (drug release, scaffolds for tissue engineering including nerve, bone, skin, cardiac tissues, dental and wound healing applications) due to their unique advantages such as high porosity, high surface area to volume ratio, sustainability, biocompatibility and biodegradability. Moreover some physical properties of nanofibers, such as surface morphology, fiber diameter, porosity etc., can be controlled, changed or mimicked easily by controlling electrospinning process parameters. In spite of these advantages, electrospinning process has some difficulties for biomedical applications. Difficulties in the production of 3D scaffolds with macro pores and scaling up into industrial scale are the major problems. In order to overcome these problems, new electrospinning-based techniques such as edge spinning, needleless electrospinning, near-field electrospinning etc., can be used for biomedical applications.
Lastly, most of the academic research related with PLA-based nanofibers has been made as in vitro and in vivo studies, and it’s very limited. Limited clinical applications is one of the obstacles to the commercialization of PLA-based medical devices, therefore more research is required on the clinical applications of PLA based fibers.
Article Highlights
Electrospinning is a versatile technology for the fabrication of nanofibers of desired fiber morphology.
Electrospinning has tremendous applications in the biomedical arena including wound healing, drug delivery, tissue engineering and dental applications.
This review encompass an overview of the development and challenges of electrospun PLA based nanofibers used in many biomedical applications
Advantages and limitations of electrospinning technique for biomedical applications are described.
Future perspectives of electrospun PLA based fibers are discussed.
Acknowledgements
This article is based upon work from COST Action AMiCI (CA15114), supported by COST (European Cooperation in Science and Technology). The authors would also like to thank Yalova University for supporting this study through a Graduate Research Project No: 2018/YL/0018.
Conflict of Interest
The author(s) declare(s) that the funding is done by author only.
Funding Source
The author(s) declare(s) that the funding is done by the author.
References
- Y. Zhang, C.T. Lim, S. Ramakrishna, Z.-M. Huang. Recent development of polymer nanofibers for biomedical and biotechnological applications, Journal of Materials Science: Materials in Medicine. 2005;16(10):933-946.
CrossRef
- Z.-M. Huang, Y.-Z. Zhang, M. Kotaki, S. Ramakrishna. A review on polymer nanofibers by electrospinning and their applications in nanocomposites, Composites science and technology. 2003;63(15):2223-2253.
CrossRef
- J.M. Deitzel, J. Kleinmeyer, D. Harris, N.C. Beck Tan. The effect of processing variables on the morphology of electrospun nanofibers and textiles, Polymer. 2001;42(1):261-272.
CrossRef
- G. Ward. Meltblown nanofibres for nonwoven filtration applications, Filtration & separation. 2001;38(9): 42-43.
CrossRef
- D.H. Reneker, I. Chun. Nanometre diameter fibres of polymer, produced by electrospinning, Nanotechnology. 1996;7(3):216.
CrossRef
- P.X. Ma, R. Zhang. Synthetic nano-scale fibrous extracellular matrix. 1999.
- F. Yang, R. Murugan, S. Ramakrishna, X. Wang, Y.-X. Ma, S. Wang. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering, Biomaterials. 2004;25(10):1891-1900.
CrossRef
- C.R. Martin. Membrane-based synthesis of nanomaterials, Chemistry of Materials. 1996;8(8):1739-1746.
CrossRef
- L. Feng, S. Li, H. Li, J. Zhai, Y. Song, L. Jiang, D. Zhu. Super‐hydrophobic surface of aligned polyacrylonitrile nanofibers, Angewandte Chemie International Edition. 2002;41(7):1221-1223.
CrossRef
- J.D. Hartgerink, E. Beniash, S. I. Stupp. Self-assembly and mineralization of peptide-amphiphile nanofibers, Science. 2001;294(5547):1684-1688.
CrossRef
- N.G. Rim, C.S. Shin, H. Shin. Current approaches to electrospun nanofibers for tissue engineering, Biomedical materials. 2013;8(1):014102.
CrossRef
- D. Kai, S. S. Liow, X. J. Loh. Biodegradable polymers for electrospinning: towards biomedical applications, Materials Science and Engineering: C. 2014;45:659-670.
CrossRef
- P.P. Sushant Upadhyaya, Rajesh Verma. Biodegradable polymer for biomedical applications, Materials Science Research India. 2006;3(24):283-285.
- İ. Esentürk, M.S. Erdal, S. Güngör. Electrospinning method to produce drug-loaded nanofibers for topical/transdermal drug delivery applications,
- J. Pelipenko, P. Kocbek, J. Kristl. Critical attributes of nanofibers: preparation, drug loading, and tissue regeneration, International journal of pharmaceutics. 2015;484(1-2):57-74.
- D. Li, M.W. Frey, A.J. Baeumner. Electrospun polylactic acid nanofiber membranes as substrates for biosensor assemblies, Journal of Membrane Science. 2006;279(1-2):354-363.
CrossRef
- M. Bognitzki, W. Czado, T. Frese, A. Schaper, M. Hellwig, M. Steinhart, A. Greiner, J.H. Wendorff. Nanostructured fibers via electrospinning, Advanced Materials. 2001;13(1):70-72.
CrossRef
- X. Zong, K. Kim, D. Fang, S. Ran, B.S. Hsiao, B. Chu. Structure and process relationship of electrospun bioabsorbable nanofiber membranes, Polymer. 2002;43(16):4403-4412.
CrossRef
- R. Casasola, N.L. Thomas, A. Trybala, S. Georgiadou. Electrospun poly lactic acid (PLA) fibres: effect of different solvent systems on fibre morphology and diameter, Polymer. 2014;55(18):4728-4737.
CrossRef
- US5247058A. Continuous process for manufacture of lactide polymers with controlled optical purity, Cargill Inc. 1992.
- S. Ebnesajjad. Handbook of biopolymers and biodegradable plastics: properties, processing and applications, William Andrew. 2012.
- E. Yalcin, F. Cicek, K. Cavusoglu. Siklodekstrin Bağlı Poli (Laktid-Ko-Glikolid) Mikropartikullerinin Sentezi, Karakterizasyonu, In vitro Kolesterol Gideriminde Kullanılabilirligi, Cumhuriyet Universitesi Fen-Edebiyat Fakultesi Fen Bilimleri Dergisi. 2013;35(3):9-22.
- T.T.T. Nguyen, C. Ghosh, S.-G. Hwang, L. Dai Tran, J.S. Park. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing, Journal of Materials Science. 2013;48(20):7125-7133.
CrossRef
- J. Li, Y. Hu, T. He, M. Huang, X. Zhang, J. Yuan, Y. Wei, X. Dong, W. Liu, F. Ko. Electrospun Sandwich‐Structure Composite Membranes for Wound Dressing Scaffolds with High Antioxidant and Antibacterial Activity, Macromolecular Materials and Engineering. 2018;303(2):1700270.
CrossRef
- X. Liu, L.H. Nielsen, S.N. Kłodzińska, H.M. Nielsen, H. Qu, L.P. Christensen, J. Rantanen, M. Yang. Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing, European Journal of Pharmaceutics and Biopharmaceutics. 2018;123:42-49.
CrossRef
- X. Ren, Y. Han, J. Wang, Y. Jiang, Z. Yi, H. Xu, Q. Ke. An aligned porous electrospun fibrous membrane with controlled drug delivery–An efficient strategy to accelerate diabetic wound healing with improved angiogenesis, Acta Biomaterialia. 2018;70:140-153.
CrossRef
- X. Zong, H. Bien, C.-Y. Chung, L. Yin, D. Fang, B.S. Hsiao, B. Chu, E. Entcheva. Electrospun fine-textured scaffolds for heart tissue constructs, Biomaterials. 2005;26(26):5330-5338.
CrossRef
- J. Stitzel, J. Liu, S.J. Lee, M. Komura, J. Berry, S. Soker, G. Lim, M. Van Dyke, R. Czerw, J. J. Yoo. Controlled fabrication of a biological vascular substitute, Biomaterials. 2006;27(7):1088-1094.
CrossRef
- V. Bakola, V. Karagkiozaki, A. Tsiapla, F. Pappa, I. Moutsios, E. Pavlidou, S. Logothetidis. Dipyridamole-.loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents, Nanotechnology. 2018;29(27):275101.
CrossRef
- H. Koh, T. Yong, C. Chan, S. Ramakrishna, Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin, Biomaterials. 2008;29(26):3574-3582.
CrossRef
- M.P. Prabhakaran, J. Venugopal, S. Ramakrishna. Electrospun nanostructured scaffolds for bone tissue engineering, Acta Biomaterialia. 2009;5(8):2884-2893.
CrossRef
- S.R. Bhattarai, N. Bhattarai, H.K. Yi, P.H. Hwang, D.I. Cha, H.Y. Kim. Novel biodegradable electrospun membrane: scaffold for tissue engineering, Biomaterials. 2004;25(13):2595-2602.
CrossRef
- E.-R. Kenawy, G.L. Bowlin, K. Mansfield, J. Layman, D.G. Simpson, E.H. Sanders, G.E. Wnek. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend, Journal of Controlled Release. 2002;81(1-2):57-64.
CrossRef
- V. Holan, M. Chudickova, P. Trosan, E. Svobodova, M. Krulova, S. Kubinova, E. Sykova, J. Sirc, J. Michalek, M. Juklickova. Cyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppression, Journal of Controlled Release. 2011;156(3):406-412.
CrossRef
- Z. Mao, J. Li, W. Huang, H. Jiang, B.L. Zimba, L. Chen, J. Wan, Q. Wu. Preparation of poly (lactic acid)/graphene oxide nanofiber membranes with different structures by electrospinning for drug delivery, RSC Advances. 2018;8(30):16619-16625.
CrossRef
- S. Chen, Y. Hao, W. Cui, J. Chang, Y. Zhou. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis, Journal of Materials Science. 2013;48(19):6567-6577.
CrossRef
- S. Thomas. Wound management and dressings, Pharmaceutical Press. 1990.
- S.A. Eming, P. Martin, M. Tomic-Canic. Wound repair and regeneration: mechanisms, signaling, and translation, Science Translational Medicine. 2014;6(265):265sr6-265sr6.
CrossRef
- R. Chen, J.A. Hunt. Biomimetic materials processing for tissue-engineering processes, Journal of Materials Chemistry. 2007;17(38):3974-3979.
CrossRef
- S. Wani, H.S. Sofi, S. Majeed, F.A. Sheikh. Recent Trends in Chitosan Nanofibers: From Tissue-Engineering to Environmental Importance: A Review, Material Science Research India. 2017;14(2):89-99.
CrossRef
- D. Kai, M.P. Prabhakaran, G. Jin, S. Ramakrishna. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering, Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;98(2):379-386.
CrossRef
- L. Tian, M.P. Prabhakaran, X. Ding, D. Kai, S. Ramakrishna. Emulsion electrospun vascular endothelial growth factor encapsulated poly (l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering, Journal of Materials Science. 2012;47(7):3272-3281.
CrossRef
- D.A. Stout, B. Basu, T.J. Webster. Poly (lactic–co-glycolic acid): carbon nanofiber composites for myocardial tissue engineering applications, Acta Biomaterialia. 2011;7(8):3101-3112.
CrossRef
- S.A. Sell, M.J. McClure, K. Garg, P.S. Wolfe, G.L. Bowlin. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering, Advanced Drug Delivery Reviews. 2009;61(12):1007-1019.
CrossRef
- L. Ghasemi-Mobarakeh, M.P. Prabhakaran, M. Morshed, M.-H. Nasr-Esfahani, S. Ramakrishna, Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering, Biomaterials, 29(34), 4532-4539, (2008).
CrossRef
- A. Subramanian, U.M. Krishnan, S. Sethuraman. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration, Journal of Biomedical Science. 2009;16(1):108.
CrossRef
- T. Bini, S. Gao, S. Wang, S. Ramakrishna. Poly (l-lactide-co-glycolide) biodegradable microfibers and electrospun nanofibers for nerve tissue engineering: an in vitro study, Journal of Materials Science. 2006;41(19):6453-6459.
CrossRef
- F. Yang, R. Murugan, S. Wang, S. Ramakrishna. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering, Biomaterials. 2005;26(15):2603-2610.
CrossRef
- M.P. Prabhakaran, J. Venugopal, C.K. Chan, S. Ramakrishna. Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering, Nanotechnology. 2008;19(45):455102.
CrossRef
- X. Liu, L.A. Smith, J. Hu, P.X. Ma. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering, Biomaterials. 2009;30(12):2252-2258.
CrossRef
- J. Venugopal, P. Vadgama, T.S. Kumar, S. Ramakrishna. Biocomposite nanofibres and osteoblasts for bone tissue engineering, Nanotechnology. 2007;18(5):055101.
CrossRef
- M.V. Jose, V. Thomas, K.T. Johnson, D.R. Dean, E. Nyairo. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering, Acta Biomaterialia. 2009;5(1):305-315.
CrossRef
- J. Ramier, T. Bouderlique, O. Stoilova, N. Manolova, I. Rashkov, V. Langlois, E. Renard, P. Albanese, D. Grande. Biocomposite scaffolds based on electrospun poly (3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications, Materials Science and Engineering: C. 2014;38:161-169.
CrossRef
- H. Wang, Y. Li, Y. Zuo, J. Li, S. Ma, L. Cheng. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering, Biomaterials. 2007;28(22): 3338-3348.
CrossRef
- M.P. Prabhakaran, L. Ghasemi-Mobarakeh, S. Ramakrishna. Electrospun composite nanofibers for tissue regeneration, Journal of Nanoscience and Nanotechnology. 2011;11(4):3039-3057.
CrossRef
- K. Shalumon, D. Sathish, S. Nair, K. Chennazhi, H. Tamura, R. Jayakumar. Fabrication of aligned poly (lactic acid)-chitosan nanofibers by novel parallel blade collector method for skin tissue engineering, Journal of Biomedical Nanotechnology. 2012;8(3):405-416.
- D. Sundaramurthi, U.M. Krishnan, S. Sethuraman. Electrospun nanofibers as scaffolds for skin tissue engineering, Polymer Reviews. 2014;54(2):348-376.
- J.-f. Pan, N.-h. Liu, H. Sun, F. Xu, Preparation and characterization of electrospun PLCL/poloxamer nanofibers and dextran/gelatin hydrogels for skin tissue engineering, PLoS One, e112885. 2014. 9(11).
- A.S. Asran, K. Razghandi, N. Aggarwal, G.H. Michler, T. Groth. Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin, Biomacromolecules. 2010;11(12):3413-3421.
- E. J. Benjamin, S. S. Virani, C. W. Callaway, A. M. Chamberlain, A. R. Chang, S. Cheng, S. E. Chiuve, M. Cushman, F. N. Delling, R. Deo. Heart disease and stroke statistics—2018 update: a report from the American Heart Association, Circulation. 2018;137(12):e67-e492.
CrossRef
- O. Chestnov. WHO Global action plan for the prevention and control of noncommunicable diseases 2013–2020, World Health Organization. 2018.
- G. Vunjak-Novakovic, N. Tandon, A. Godier, R. Maidhof, A. Marsano, T.P. Martens, M. Radisic. Challenges in cardiac tissue engineering, Tissue Engineering Part B: Reviews. 2009;16(2):169-187.
CrossRef
- Q.-Z. Chen, S. E. Harding, N.N. Ali, A.R. Lyon, A.R. Boccaccini. Biomaterials in cardiac tissue engineering: ten years of research survey, Materials Science and Engineering: R: Reports. 2008;59(1-6):1-37.
- J. Yang, M. Yamato, T. Shimizu, H. Sekine, K. Ohashi, M. Kanzaki, T. Ohki, K. Nishida, T. Okano. Reconstruction of functional tissues with cell sheet engineering, Biomaterials. 2007;28(34):5033-5043.
- J.S. Belkas, M. S. Shoichet, R. Midha. Peripheral nerve regeneration through guidance tubes, Neurological Research. 2004;26(2):151-160.
- W. Daly, L. Yao, D. Zeugolis, A. Windebank, A. Pandit. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery, Journal of the Royal Society Interface. 2012;9(67):202-221.
- V. Mukhatyar, L. Karumbaiah, J. Yeh, R. Bellamkonda. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves, Advanced Materials. 2009;21(46):4670-4679.
- L.R. Williams, F.M. Longo, H.C. Powell, G. Lundborg, S. Varon. Spatial‐temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay, Journal of Comparative Neurology. 1983;218(4):460-470.
CrossRef
- J. Wang, L. Tian, L. He, N. Chen, S. Ramakrishna, K.-F. So, X. Mo, Lycium barbarum polysaccharide encapsulated Poly lactic-co-glycolic acid Nanofibers: cost effective herbal medicine for potential application in peripheral nerve tissue engineering, Scientific Reports. 2018;8.
- R.L. Dahlin, F.K. Kasper, A.G. Mikos. Polymeric nanofibers in tissue engineering, Tissue Engineering Part B: Reviews. 2011;17(5):349-364.
- M. Nune, S. Manchineella, T. Govindaraju, K. Narayan, Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering, Materials Science and Engineering: C. 2019;94:17-25.
- N. Saadatkish, S. Nouri Khorasani, M. Morshed, A.R. Allafchian, M.H. Beigi, M. Masoudi Rad, R. Esmaeely Neisiany, M.H. Nasr‐Esfahani. A ternary nanofibrous scaffold potential for central nerve system tissue engineering, Journal of Biomedical Materials Research Part A. 2018.
- U. Topaloglu, M.A. Ketani, B.G. Saruhan. Kemik Doku ve Kemiklesme Cesitleri, Dicle Üniversitesi Veteriner Fakultesi Dergisi. 2017;10(1):62-71.
- A.R. Amini, C.T. Laurencin, S.P. Nukavarapu. Bone tissue engineering: recent advances and challenges, Critical Reviews™ in Biomedical Engineering. 2012;40(5).
- Blecha, P. Zambelli, N. Ramaniraka, P.-E. Bourban, J.-A. Månson, D.P. Pioletti. How plate positioning impacts the biomechanics of the open wedge tibial osteotomy; a finite element analysis, Computer methods in biomechanics and biomedical engineering. 2005;8(5):307-313.
- I. Martin, T. Smith, D. Wendt. Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products, Trends in Biotechnology. 2009;27(9):495-502.
- Y. Zhang, J. R. Venugopal, A. El-Turki, S. Ramakrishna, B. Su, C.T. Lim. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering, Biomaterials. 2008;29(32):4314-4322.
- M.E. Frohbergh, A. Katsman, G. P. Botta, P. Lazarovici, C. L. Schauer, U.G. Wegst, P.I. Lelkes. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering, Biomaterials. 2012;33(36):9167-9178.
- J. Venugopal, S. Low, A.T. Choon, T.S. Kumar, S. Ramakrishna. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers, Journal of Materials Science: Materials in Medicine. 2008;19(5):2039-2046.
- L. Lao, Y. Wang, Y. Zhu, Y. Zhang, C. Gao. Poly (lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering, Journal of Materials Science: Materials in Medicine. 2011;22(8):1873-1884.
- C. Erisken, D.M. Kalyon, H. Wang. Functionally graded electrospun polycaprolactone and β-tricalcium phosphate nanocomposites for tissue engineering applications, Biomaterials. 2008;29(30):4065-4073.
CrossRef
- M. Ngiam, S. Liao, A.J. Patil, Z. Cheng, C.K. Chan, S. Ramakrishna, The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering, Bone. 2009;45(1):4-16.
- J.H. Song, B.H. Yoon, H.E. Kim, H.W. Kim. Bioactive and degradable hybridized nanofibers of gelatin–siloxane for bone regeneration, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2008;84(4):875-884.
- R. Sedghi, N. Sayyari, A. Shaabani, H. Niknejad, T. Tayebi. Novel biocompatible zinc-curcumin loaded coaxial nanofibers for bone tissue engineering application, Polymer. 2018;142:244-255.
- C. Zhu, J. Zhu, C. Wang, R. Chen, L. Sun, C. Ru. Wrinkle-Free, Sandwich, Electrospun PLGA/SF Nanofibrous Scaffold for Skin Tissue Engineering, IEEE Transactions on Nanotechnology. 2018.
- Z. Lin, W. Gao, L. Ma, H. Xia, W. Xie, Y. Zhang, X. Chen. Preparation and properties of poly (ε-caprolactone)/bioactive glass nanofibre membranes for skin tissue engineering, Journal of Bioactive and Compatible Polymers. 2018;33(2):195-209.
- B. Dhandayuthapani, U.M. Krishnan, S. Sethuraman. Fabrication and characterization of chitosan‐gelatin blend nanofibers for skin tissue engineering, Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;94(1):264-272.
- S. Gautam, C.-F. Chou, A.K. Dinda, P.D. Potdar, N.C. Mishra. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering, Materials Science and Engineering: C. 2014;34:402-409.
- N. Bhardwaj, S.C. Kundu. Electrospinning: a fascinating fiber fabrication technique, Biotechnology Advances. 2010;28(3):325-347.
- V. Leung, F. Ko. Biomedical applications of nanofibers, Polymers for Advanced Technologies. 2011;22(3): 350-365.
CrossRef
- P. Taepaiboon, U. Rungsardthong, P. Supaphol. Drug-loaded electrospun mats of poly (vinyl alcohol) fibres and their release characteristics of four model drugs, Nanotechnology. 2006;17(9):2317.
- J. Zeng, L. Yang, Q. Liang, X. Zhang, H. Guan, X. Xu, X. Chen, X. Jing. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation, Journal of Controlled Release. 2005;105(1-2):43-51.
- C. He, W. Nie, W. Feng. Engineering of biomimetic nanofibrous matrices for drug delivery and tissue engineering, Journal of Materials Chemistry B. 2014;2(45):7828-7848.
- R. Langer, N.A. Peppas. Advances in biomaterials, drug delivery, and bionanotechnology, AIChE Journal. 2003;49(12):2990-3006.
- S. Fredenberg, M. Wahlgren, M. Reslow, A. Axelsson. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review, International journal of pharmaceutics. 2011;415(1-2):34-52.
- K. El-Refaie, I.A.-H. Fouad, H.E.-N. Mohamed, E.W. Gary. Controlled release of ketoprofen from electrospun poly(vinyl alcohol) nanofibers, Materials Science and Engineering: A. 2007;459(1):390 – 396.
- R.I. Reda, M.M. Wen, A.H. El-Kamel. Ketoprofen-loaded Eudragit electrospun nanofibers for the treatment of oral mucositis, International journal of nanomedicine. 2017;12:2335-2351.
- D. Yu, X. Wang, X. Li, W. Chian, Y. Li, Y. Liao. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers, Acta Biomaterialia. 2013;9(3):5665-5672.
- B.L. Pihlstrom, B.S. Michalowicz, N.W. Johnson. Periodontal diseases, The Lancet. 2005;366(9499):1809-1820.
- Y. Yamada, M. Ueda, H. Hibi, S. Baba. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report, International Journal of Periodontics & Restorative Dentistry. 2006;26(4).
- H.F. Dvorak, T.M. Sioussat, L.F. Brown, B. Berse, J.A. Nagy, A. Sotrel, E.J. Manseau, L. Van De Water, D.R. Senger. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels, Journal of Experimental Medicine. 1991;174(5):1275-1278.
- S. Nyman, J. Gottlow, T. Karring, J. Lindhe. The regenerative potential of the periodontal ligament: an experimental study in the monkey, Journal of Clinical Periodontology. 1982;9(3);257-265.
- S. Nyman, J. Gottlow, J. Lindhe, T. Karring, J. Wennstrom. New attachment formation by guided tissue regeneration, Journal of Periodontal Research. 1987;22(3):252-254.
- G. Polimeni, K.T. Koo, G.A. Pringle, A. Agelan, F.F. Safadi, U.M. Wikesjö. Histopathological observations of a polylactic acid‐based device intended for guided bone/tissue regeneration, Clinical implant dentistry and related research. 2008;10(2):99-105.
- I. Magnusson, C. Batich, B. Collins, New attachment formation following controlled tissue regeneration using biodegradable membranes, Journal of periodontology. 1988;59(1):1-6.
- C. C. Villar, D. L. Cochran, Regeneration of periodontal tissues: guided tissue regeneration, Dental Clinics. 2010;54(1):73-92.
- M. Simion, M. Baldoni, P. Rassi, D. Zaffe. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period, International Journal of Periodontics & Restorative Dentistry. 1994;14(2).
- D. Rothamel, F. Schwarz, M. Sager, M. Herten, A. Sculean, J. Becker. Biodegradation of differently cross‐linked collagen membranes: an experimental study in the rat, Clinical oral implants research. 2005;16(3): 369-378.
- K. A. Athanasiou, G. G. Niederauer, C. M. Agrawal. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers, Biomaterials. 1996;17(2):93-102.
- P. Trejo, R. Weltman, R. Caffesse. Effects of expanded polytetrafluoroethylene and polylactic acid barriers on healthy sites, Journal of Periodontology. 1998;69(1):14-18.

This work is licensed under a Creative Commons Attribution 4.0 International License.

 Material Science Research India An International Peer Reviewed Research Journal
Material Science Research India An International Peer Reviewed Research Journal