Published online Dec 6, 2021. doi: 10.12998/wjcc.v9.i34.10689
Peer-review started: April 23, 2021
First decision: June 25, 2021
Revised: July 7, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: December 6, 2021
In the context of aortic dissection, increasing pressure within the newly formed false lumen can result in the progressive compression of the true aortic channel. However, true lumen collapse in chronic type B aortic dissection (cTBAD) patients is rare, with few clinical or experimental studies to date having explored the causes of such collapse.
In the present report, we describe a rare case of true-lumen collapse in an 83-year-old patient diagnosed with cTBAD, and we discuss potential therapeutic interventions for such cases. Following thoracic endovascular aortic repair (TEVAR), computed tomography angiography revealed satisfactory stent-graft positioning, no endoleakage, true lumen enlargement, thrombus formation in the false lumen, and slight enlargement of the true lumen distal to the stent-graft. Computational hemodynamic analyses indicated that the wall shear stress and pressure within the false lumen were significantly reduced following TEVAR.
TEVAR treatment of cTBAD patients suffering from proximal true lumen collapse can facilitate some degree of effective remodeling.
Core Tip: We describe a rare case of true-lumen collapse in an 83-year-old patient with chronic type B aortic dissection, and we discuss potential therapeutic interventions for such cases.
- Citation: Zhang L, Guan WK, Wu HP, Li X, Lv KP, Zeng CL, Song HH, Ye QL. Proximal true lumen collapse in a chronic type B aortic dissection patient: A case report. World J Clin Cases 2021; 9(34): 10689-10695
- URL: https://www.wjgnet.com/2307-8960/full/v9/i34/10689.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i34.10689
Intimal aortic tears that ultimately cause aortic dissection can allow blood to flow between the medial layers of the dissected aorta, leading to the formation of a double-barreled aortic lumen. Over time, increasing pressure within the newly formed false lumen can result in the progressive compression of the true aortic channel. Studies in experimental model systems suggest that the incidence of true-lumen collapse is strongly dependent upon the differential between the inflow : outflow capacity ratios of the true lumen and the false lumen, with this incidence further being influenced by other physiologic and anatomic factors. The definitive treatment for such cases of true lumen collapse is the targeted repair of the entry tear as a means of reducing inflow into the false lumen[1,2]. True lumen collapse in chronic type B aortic dissection (cTBAD) patients is rare, with few clinical or experimental studies to date having explored the causes of true lumen collapse in this context. Optimal treatment methods for patients suffering from this rare condition are similarly not well defined. In the present report, we describe the case of a cTBAD patient who suffered from true lumen collapse. Using computed tomography angiography (CTA) data from this patient collected at both initial presentation and at 3 and 28 mo post-treatment, we additionally develop computational models to observe hemodynamic changes before and after thoracic endovascular aortic repair (TEVAR).
An 83-year-old male was admitted to our hospital due to the incidental detection of an aortic dissection aneurysm during a standard health examination.
The patient did not exhibit any chest or back pain and was incidentally diagnosed with an aortic dissection aneurysm while undergoing a routine health evaluation.
The patient had previously been diagnosed with hepatic and renal cysts.
The patient had no remarkable personal or family history.
The arteries of the extremities exhibited normal pulsatile activity, and all limbs exhibited normal motor and sensory functions. There was also no evidence of peritonitis.
No abnormality.
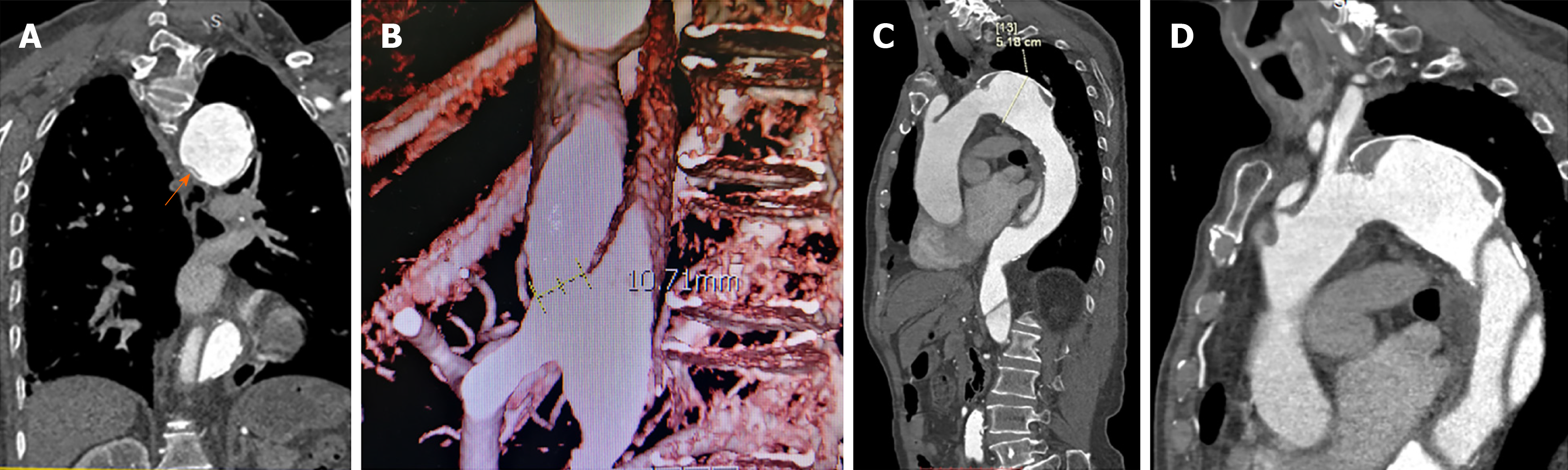
CTA confirmed the dissection of the descending aorta and aortic arch. A dilated false lumen with evidence of calcification was visible as was the collapse of the proximal true lumen. The aortic dissection had a maximal diameter of 51.8 mm, with no tearing of the thoracic aorta. The distal tear was located on the common trunk of the celiac axis and the superior mesenteric artery and had a maximal diameter of 10.71 mm (Figure 1).
CTBAD with proximal true lumen collapse, liver cyst, renal cyst.
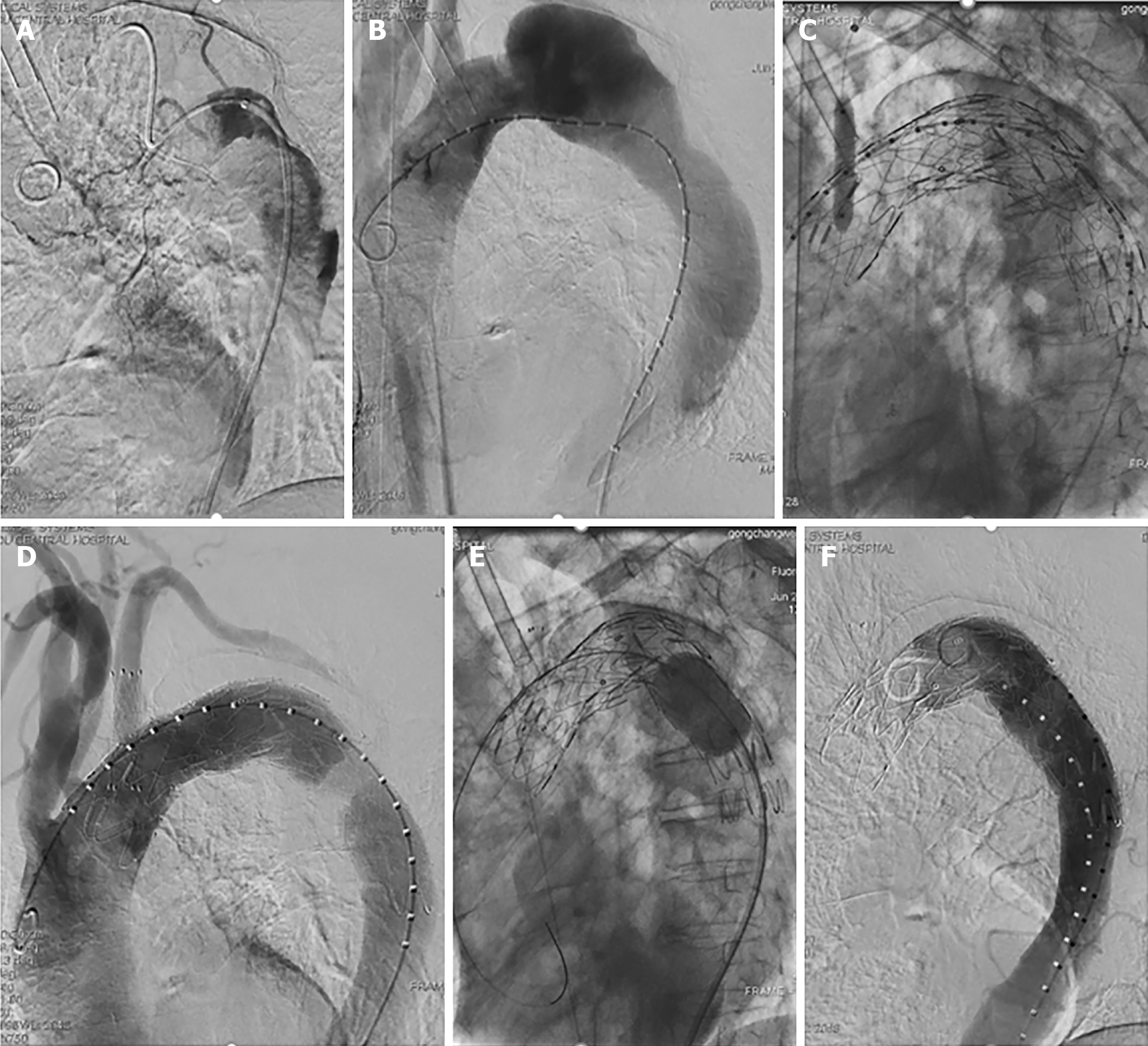
Significant compression of the proximal true lumen was evident in an aortogram, and the catheter used for this intervention had a helical shape (Figure 2A) that was corrected using a Lunderquist guidewire (Figure 2B). In order to achieve true lumen enlargement without causing endoleakage, upper limb ischemia, or stroke, we employed a left subclavian arterial fenestration approach to conduct TEVAR. Owing to differences in the diameter of the aortic arch and the descending aorta, we used two ANKURA® stent-grafts (Lifetech Scientific Co., Ltd., Shenzhen, China). These stent-grafts (34-30 mm and 30-26 mm diameter, 120 mm long) were deployed from the distal to the left common carotid artery to the descending aorta such that they overlapped one another. The sharpened end of a V18 wire was then used to fenestrate the ANKURA stent graft, with this hole being enlarged through the use of a balloon dilatation catheter (INVATEC 3.5-120 mm and 8-40 mm, Medtronic, Inc., Dublin, Ireland), after which we placed the Fluency stent (10 mm in diameter, 40 mm in length) into the ANKURA stent graft. Balloon dilatation of the stent-graft was then performed. Subsequent angiography did not show any evidence of endoleakage, with satisfactory left subclavian artery blood blow (Figure 2C and D). A Coda balloon (Cook Medical, Bloomington, IN, United States) was used to expand the aortic stent overlap, and the stent shape was satisfactory upon subsequent angiographic asse
On day 5 post-treatment, the patient was discharged without any incidence of paraplegia, neurological abnormalities, or other serious adverse events. At 3- and 28-mo post-surgery, the patient underwent routine physical examination and CTA. CTA imaging data in the DICOM format were imported into the Mimics 19.0 software (Materialise Inc., Leuven, Belgium) for three-dimensional model reconstruction and associated measurements. Data produced by Stefanov et al[3] were used to guide hemodynamic analyses of the cardiac cycle.
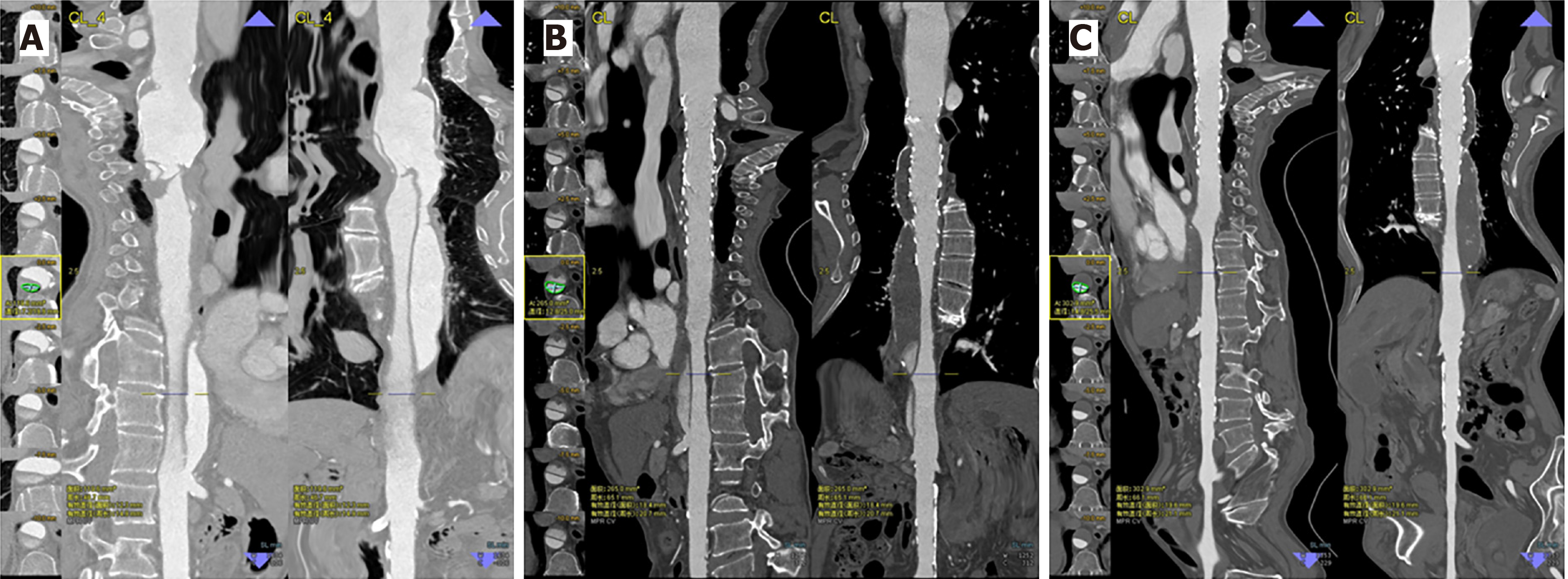
Following TEVAR, CTA results indicated that the true lumen was significantly enlarged in the stented region, while the false lumen gradually decreased in size and became completely thrombotic (Figure 3). These results thus indicate that following TEVAR the maximal descending aortic diameter grew smaller, whereas the diameter and area of the true lumen increased (Table 1).
| Maximal diameter of descending aorta | Diameter and area of the true lumen at the level of the 11th thoracic vertebra | Diameter and area of the true lumen at the end of the stent | |
| Prior to TEVAR | 51.8 mm | 7.2/19.9 mm119.6 mm2 | |
| 3-mo post-treatment | 50.8 mm | 12.8/25.0 mm265.0 mm2 | 16.8/27.0 mm349.8 mm2 |
| 28-mo post-treatment | 50.5 mm | 14.8/25.5 mm302.9 mm2 | 18.0/27.3 mm376.9mm2 |
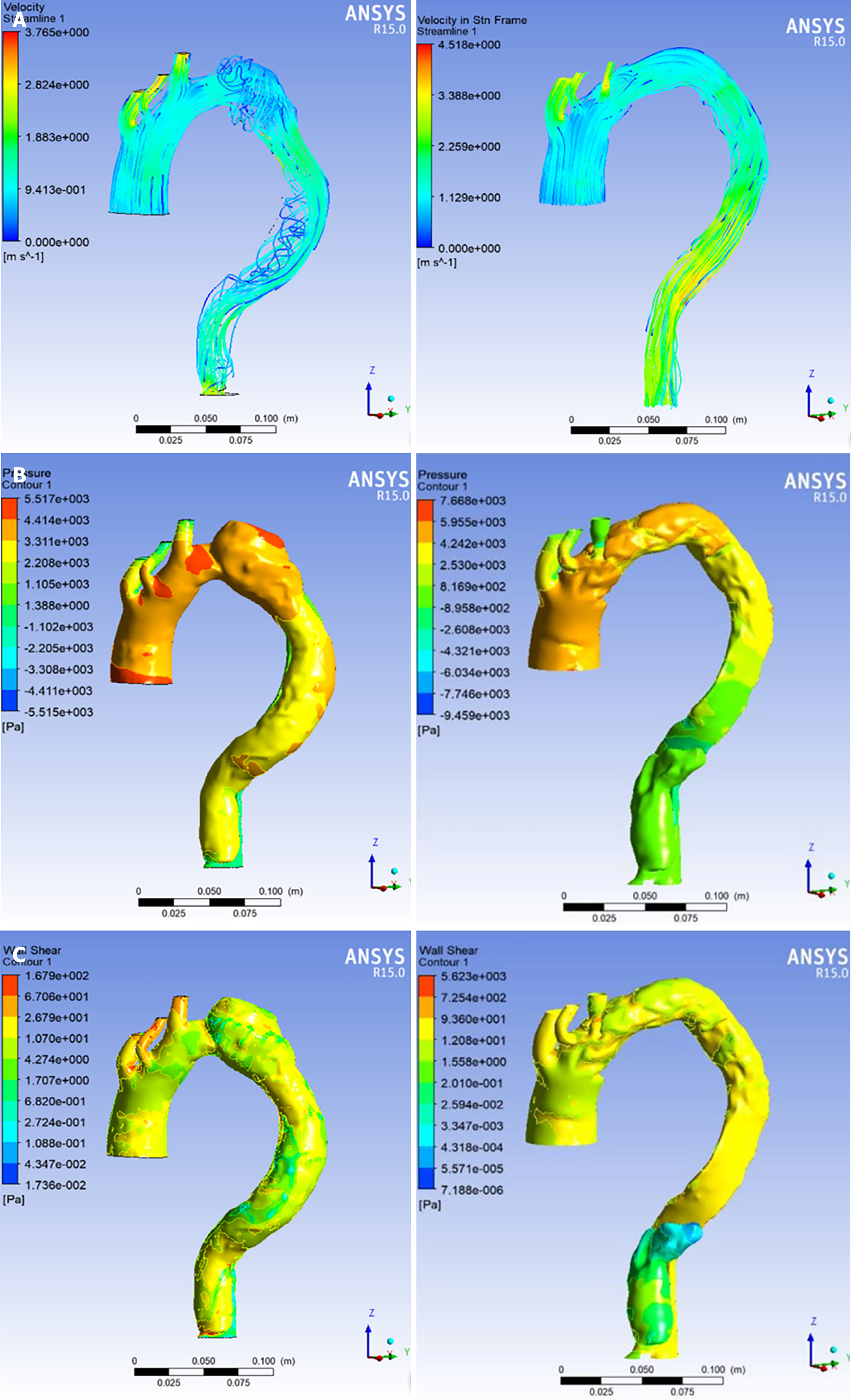
Prior to TEVAR, flow within the false lumen exhibited helical features, and pressure and wall shear stress values were significantly higher in the false lumen relative to the true lumen, with these changes being most prominent in the enlarged region of the false lumen. Following TEVAR, there was no evidence of helical flow within the false lumen. In addition, treatment was associated with significant reductions in both pressure and wall shear stress within the false lumen and significant increases in these values in the true lumen, with these pressure values being nearly equal proximal to the tear (Figure 4).
Following the publication of the 2014 European Society of Cardiology guidelines[4], preemptive TEVAR has now received broad approval as the treatment of choice for patients diagnosed with subacute uncomplicated type B aortic dissection. Treatment of these patients is recommended in order to reduce the risk of aortic expansion, rupture, and recurrent dissection. Even following treatment, between 20% and 40% of these patients will need a secondary operation for the treatment of aortic aneurysmal degeneration[5]. Favorable aortic remodeling above the celiac artery has been observed following TEVAR treatment, with no differences in remodeling outcomes being observed between acute and chronic complicated type B aortic dissection patients[6]. Favorable early and mid-term outcomes have previously been observed following the endovascular treatment of type B chronic aneurysmal aortic dissection[7], and one or more additional procedures can achieve a good long-term result[8]. Secondary aortic intervention after TEVAR for type B aortic dissection does not affect survival[9]. Even so, these past findings suggest that endovascular treatment the TEVAR-based treatment of proximal true-lumen collapse in a cTBAD patient could be considered to be one of the effective treatment options. Following TEVAR, the false lumen in this patient gradually underwent complete thrombosis, the maximal descending aortic diameter decreased, and the size and diameter of the true lumen in both the graft and distal regions increased, consistent with the work of Chung et al[2]. For patients with symptomatic aortic dissection with true lumen collapse, endovascular fenestration of the dissection flap and/or expansion of the true lumen with a large-diameter balloon can be used to increase the perfusion of the true lumen, as demonstrated in previously published case reports[10,11].
Few anatomic predictors of true lumen collapse had been identified to date, with true lumen size at admission being the factor most strongly associated with such collapse or malperfusion, as it typically corresponds to significant changes in false lumen blood flow as measured via computational hemodynamic analyses[12]. Such computational hemodynamic approaches have been used with increasing frequency as a means of assessing cases of aortic dissection, as both pressure and wall shear stress are key parameters associated with dissection onset and progression. Importantly, lower levels of wall shear stress in the false lumen can promote local thrombosis[13]. In the present case, we observed high pressure within the proximal false lumen that was associated with an impinging jet of high-pressure blood flow along the outer wall of this false lumen. This jet impingement was also associated with a visible local increase in the aortic diameter. Following the TEVAR treatment of this patient, we observed complete thrombosis of the false lumen with only a small percentage of blood flow still entering into this compartment. This was associated with higher pressure in the true lumen relative to the false lumen unlike the near-equal pressure values observed before treatment, thereby reversing this factor associated with luminal collapse. Prior to TEVAR, we observed clear evidence of higher shear wall stress in the proximal false lumen relative to the true lumen, whereas this was reversed following treatment. Our computational analyses further confirmed that reductions in pressure and wall shear stress may facilitate positive aortic wall remodeling.
While these findings are promising, this study is limited by the fact that it is a description of a single case. Future studies that expand upon these results in a larger patient cohort are essential in order to define more conclusively optimal treatment strategies in similar cases.
The findings from the present case suggest that TEVAR can be effectively implemented as a means of treating proximal true-lumen collapse in cTBAD patients. This treatment strategy achieved good efficacy and was not associated with any significant neurological complications, stent-related complications, or other adverse events. Follow-up data from this patient indicated that TEVAR induced some degree of remodeling when used to treat cTBAD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jesus-Silva SG, Schoenhagen P S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yu HG
| 1. | Chung JW, Elkins C, Sakai T, Kato N, Vestring T, Semba CP, Slonim SM, Dake MD. True-lumen collapse in aortic dissection: part I. Evaluation of causative factors in phantoms with pulsatile flow. Radiology. 2000;214:87-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Chung JW, Elkins C, Sakai T, Kato N, Vestring T, Semba CP, Slonim SM, Dake MD. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology. 2000;214:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Stefanov F, Sultan S, Morris L, Elhelali A, Kavanagh EP, Lundon V, Sultan M, Hynes N. Computational fluid analysis of symptomatic chronic type B aortic dissections managed with the Streamliner Multilayer Flow Modulator. J Vasc Surg. 2017;65:951-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, John Manolis A, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, von Allmen RS, Vrints CJ; Authors/Task Force members. Corrigendum to: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2015;36:2779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Durham CA, Cambria RP, Wang LJ, Ergul EA, Aranson NJ, Patel VI, Conrad MF. The natural history of medically managed acute type B aortic dissection. J Vasc Surg. 2015;61:1192-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Chou HW, Chan CY, Chang CH, Lin CF, Chen YS, Wang SS, Wu IH. Comparisons of aortic remodelling and outcomes after endovascular repair of acute and chronic complicated Type B aortic dissections. Interact Cardiovasc Thorac Surg. 2018;27:733-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kanaoka Y, Ohki T, Kurosawa K, Maeda K, Shukuzawa K, Hara M, Baba T, Takizawa R, Tachihara H. Early and midterm outcomes of endovascular treatment for chronic aneurysmal aortic dissection: a retrospective study. Ther Adv Cardiovasc Dis. 2018;12:275-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Puech-Leao P, Estenssoro AEV, Wakassa TB, Casella IB, DeLuccia N. Long-term Results of Endovascular Treatment of Chronic Type B Aortic Dissection by Closure of the Primary Tear. Ann Vasc Surg. 2020;66:179-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Giles KA, Beck AW, Lala S, Patterson S, Back M, Fatima J, Arnaoutakis DJ, Arnaoutakis GJ, Beaver TM, Berceli SA, Upchurch GR, Huber TS, Scali ST. Implications of secondary aortic intervention after thoracic endovascular aortic repair for acute and chronic type B dissection. J Vasc Surg. 2019;69:1367-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Raupach J, Chovanec V, Kozakova V, Vojacek J. Endovascular fenestration of aortic dissection membrane after failed frozen elephant trunk procedure. Eur J Cardiothorac Surg. 2020;57:598-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Konstantinou N, Banafsche R, Mehmedovic A, Spanos K, Rantner B, Tsilimparis N. Balloon-Assisted True Lumen Expansion and Fenestration of a Symptomatic, Triple-Barrel, Postdissection Thoracoabdominal Aneurysm with Collapsed True Lumen to Facilitate Endovascular Treatment with a t-Branch. Ann Vasc Surg. 2021;74:521.e15-521.e21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | D'Ancona G, Lee JJ, Pasta S, Pilato G, Rinaudo A, Follis F, Pilato M. Computational analysis to predict false-lumen perfusion and outcome of type B aortic dissection. J Thorac Cardiovasc Surg. 2014;148:1756-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Menichini C, Cheng Z, Gibbs RG, Xu XY. Predicting false lumen thrombosis in patient-specific models of aortic dissection. J R Soc Interface. 2016;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |