Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10172
Peer-review started: July 7, 2021
First decision: July 26, 2021
Revised: August 12, 2021
Accepted: October 14, 2021
Article in press: October 14, 2021
Published online: November 26, 2021
Secondary hyperparathyroidism (SHPT) is a common complication in patients with end-stage renal disease and it is also common in hemodialysis patients. SHPT can increase bone fragility and calcification of blood vessels and soft tissues, which greatly increases the risk of death.
To discuss the outcome, safety and other potential benefits of paricalcitol injection in hemodialysis patients with SHPT.
We recruited 40 patients who received hemodialysis at our hospital for chronic renal failure with SHPT between March and December 2019. They received paricalcitol injection for 24 wk (starting dose, 0.06–0.08 μg/kg), three times per week. They were followed up at the baseline (week 0), week 4, week 12 and week 24. The primary outcome indicator was the percentage of patients with a > 30% decrease in intact parathyroid hormone (iPTH) levels at week 24 compared with the baseline. The secondary outcome indicators included percentage decrease in iPTH levels at week 24, standard-reaching rate of iPTH (percentage of patients with iPTH down to 130–585 pg/mL), changes in serum levels of calcium (Ca), phosphate (P), Ca × P product, alkaline phosphatase (ALP), creatinine (Cre), hemoglobin (Hb), and C-reactive protein (CRP), and incidence of adverse events (AEs).
After 24 wk of treatment, iPTH levels decreased significantly (598.88 ± 381.29 pg/mL vs 888.84 ± 376.88 pg/mL, P < 0.05). More than 30% decrease of iPTH was found in 21 of 36 (58.33%) patients. The average decrease in iPTH levels was 32.16 ± 4.33%; the standard-reaching rate of iPTH levels was 66.67% (24/36); and ALP levels decreased significantly compared with the baseline (113.72 ± 41.73 IU/L vs 133.45 ± 56.86 IU/L) (t = 2.798, P < 0.05). There were no significant differences in the serum levels of calcium, Hb, Cre and CRP compared with the baseline (P > 0.05). After 24 wk of treatment, serum P levels decreased compared with the baseline (1.91 ± 0.40 mmol/L vs 2.16 ± 0.66 mmol/L) (t = 2.830, P < 0.05). Ca × P product decreased significantly compared with the baseline (56.38 ± 13.22
Paricalcitol was a safe and effective treatment for hemodialysis patients with SHPT. It decreased serum levels of iPTH, ALP and P and maintained stability of serum Ca levels.
Core Tip: In this study, 40 patients with chronic renal failure were treated with paricalcitol for 24 wk. It was found that paricalcitol can significantly reduce intact parathyroid hormone, alkaline phosphatase and serum phosphate levels, and maintain a relatively stable serum calcium level. Therefore, paricalcitol is effective and safe in the treatment of hemodialysis patients with secondary hyperparathyroidism.
- Citation: Chen X, Zhao F, Pan WJ, Di JM, Xie WN, Yuan L, Liu Z. Paricalcitol in hemodialysis patients with secondary hyperparathyroidism and its potential benefits. World J Clin Cases 2021; 9(33): 10172-10179
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10172
Secondary hyperparathyroidism (SHPT) is a common complication in patients with end-stage renal disease. Hyperphosphatemia, hypocalcemia and 1,25(OH)2D de
We recruited 40 patients with chronic renal failure complicated by SHPT and receiving hemodialysis at our hospital between March and December 2019. There were 23 men and 17 women, with an average age of 49.10 ± 12.86 years. Inclusion criteria: (1) Age > 18 years; (2) Regular hemodialysis for ≥ 3 mo, three times per week, and hemodialysis continued during medication; (3) iPTH levels > 300 pg/mL; (4) Life expectancy > 6 mo; and (5) Good adherence to treatment. Exclusion criteria: (1) History of paricalcitol treatment before enrollment; (2) History of treatment with other active forms of vitamin D and its analogs (including calcitriol, alfacalcidol, doxercalciferol, fluorocalcidol and maxacalcitol) and calcimimetics (cinacalcet); (3) Hypercalcemia or Ca × P product > 65 mg2 /dL2; (4) Allergic to the investigational drug; (5) Serious heart disease, liver injury, active inflammatory disease, or malignancy; (6) Ready for kidney transplantation or parathyroidectomy; (7) Pregnant or lactating women; (8) Unwilling to take effective contraceptive measures; and (9) Participating in other studies in the same period. The present study was approved by the Ethics Review Committee of the hospital. All patients were enrolled on a voluntary basis and gave signed informed consent.
The investigational drug was paricalcitol injection (Zemplar®; Jiangsu Hengrui Medicine Co. Ltd., strength 1 mL: 5 μg) and stored at 30 °C. The starting dose of the paricalcitol injection was 0.06–0.08 μg/kg. Within 30 min before the end of the hemodialysis, paricalcitol injection was administered via the hemodialysis venous catheter (venous port) three times per week. The dose was adjusted according to the serum levels of iPTH, Ca and P, which were detected once every 2-4 wk. The specific dose adjustment criteria are shown in Table 1. If hypercalcemia occurred or the corrected Ca × P product was continuously above 65 mg2/dL2, the dose should be reduced or discontinued until the above parameters returned to normal. After that, paricalcitol administration was resumed starting at a lower dose. The treatment lasted for 24 wk. Follow-up was conducted at the baseline and at weeks 4, 12 and 24. The patients were followed up on all designated days, with a window period of ± 4 d.
| iPTH level compared with baseline | Dose adjustment of paricalcitol |
| Not reaching the standard, unchanged or increased; or decreased by < 30% | Increase dose by 2-4 μg |
| When 150-300 pg/mL or iPTH down by ≥ 30% | Maintain original dose |
| When iPTH < 150 pg/mL or serum Ca > 11.0 mg/mL or Ca × P product > 70 mg2/dL2 | Decrease dose by 2-4 μg |
Primary outcome indicator: Percentage of patients with > 30% decrease in iPTH levels at week 24 compared with the baseline. Secondary outcome indicators: Decrease in iPTH levels at week 24; standard-reaching rate of iPTH (percentage of patients with iPTH down to 130–585 pg/mL)[10]; changes in serum levels of Ca, P, Ca × P, alkaline phosphatase (ALP), creatinine (Cre), hemoglobin (Hb), and C-reactive protein (CRP); adverse events (AEs). The occurrence of any AEs during treatment was closely observed.
SPSS 19.0 software was used for data analysis. Measurement data (obeying normal distribution) were expressed as mean ± SD. Comparisons between the measurements at the baseline and at each time point of follow-up were conducted using the paired t test. Counts were described by cases (percentages) and subjected to Pearson’s χ2 test. P < 0.05 indicated a significant difference.
A total of 40 patients were recruited, including 23 men and 17 women. Thirty-six patients finished all treatments planned, and four were lost to follow-up (Table 2).
| Variable | Patients |
| Age in yr | 49.10 ± 12.86 |
| Sex | |
| Male | 23 (57.5%) |
| Female | 17 (42.5%) |
| Duration in mo of dialysis | 55.20 ± 29.32 |
| Weekly dose of paricalcitol in g/wk | 12.38 ± 2.77 |
| iPTH in pg/mL | 888.84 ± 376.88 |
| ALP in IU/L | 133.45 ± 56.86 |
| Blood P in mmol/L | 2.16 ± 0. 66 |
| Blood Ca in mmol/L | 2.38 ± 0.16 |
| Ca P product in mg2/dL2 | 63.97 ± 20.30 |
| Hb in g/L | 114.82 ± 20.45 |
| Cre in mol/L | 807.43 ± 254.64 |
| CRP in mg/L | 8.60 ± 16.76 |
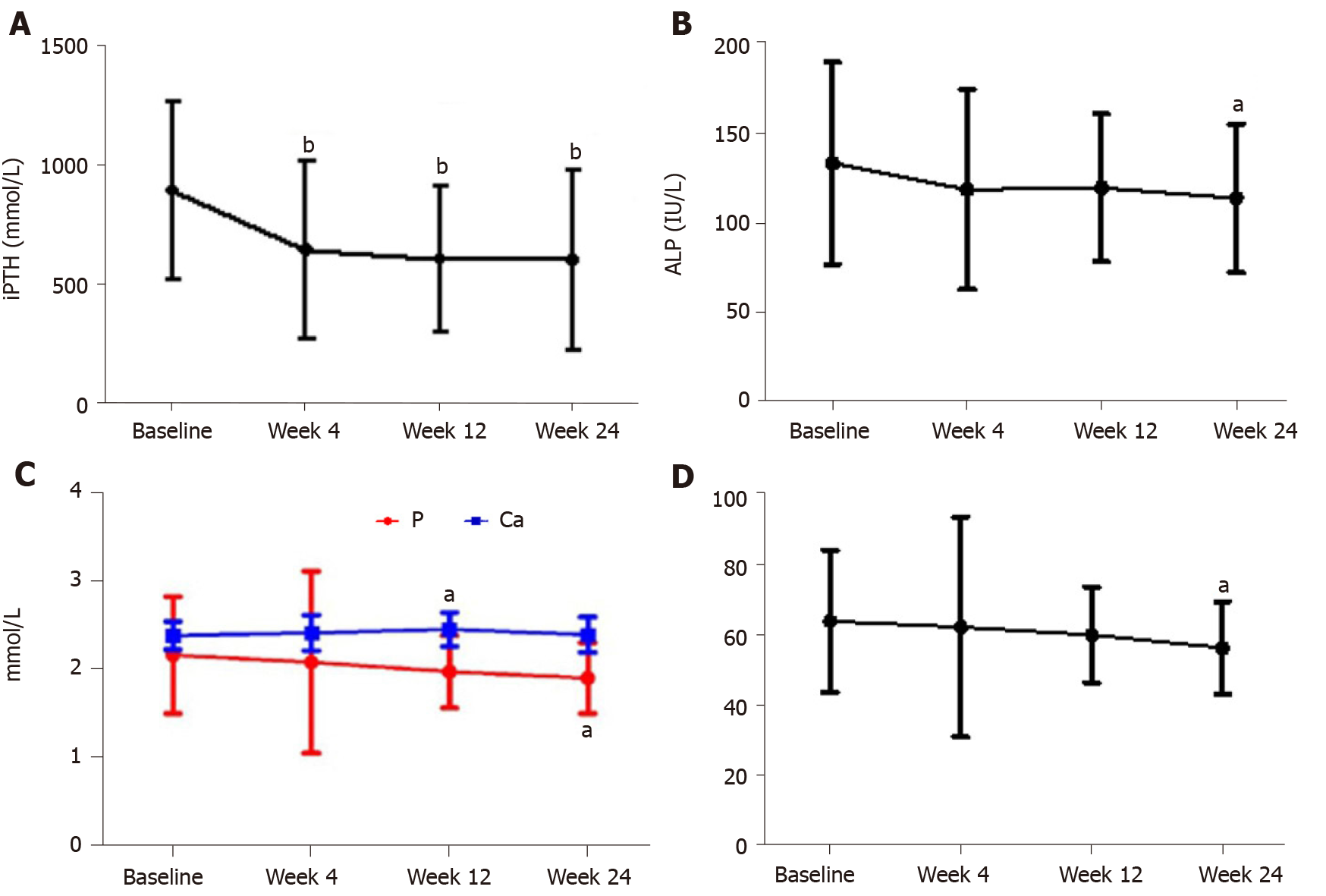
The baseline iPTH level was 888.84 ± 376.88 pg/mL. After 24 wk of treatment, it decreased to 598.88 ± 381.29 pg/mL, and the average decrease was 32%, indicating a significant difference (t = 4.589, P < 0.05) (Figure 1A). After 24 wk of treatment, 21/36 patients (58.33%) had a > 30% decrease in iPTH levels. The standard-reaching rate of iPTH was 24/36 (66.67%).
After 24 wk of treatment, ALP levels decreased significantly compared with the baseline (113.72 ± 41.73 IU/L vs 133.45 ± 56.86 IU/L) (t = 2.401, P < 0.05) (Figure 1B).
During treatment, serum Ca levels remained stable. At week 12, the serum Ca level increased to 2.45 ± 0.19 mmol/L, but was still within the normal range (2.1–2.5 mmol/L). At week 24, the serum Ca level (2.39 ± 0.20 mmol/L) was not significantly different from that at the baseline (2.38 ± 0.16 mmol/L) (t = 0.242, P > 0.05). At week 24, the serum P level (1.91 ± 0.40 mmol/L) was not significantly different from that at the baseline (2.16 ± 0.66 mmol/L) (t = 2.830, P < 0.05). At week 24, Ca × P product (56.38 ± 13.22 mg2/dL2) was not significantly different from that at the baseline (63.97 ± 20.30 mg2/dL2) (t = 2.717, P < 0.05) (Figure 1C and D).
At each time point of follow-up, there were no significant differences in Hb and CRP levels compared with the baseline (P > 0.05). At weeks 4 and 24, the Cre level was not significantly different from that at the baseline (P > 0.05). However, there was a significant difference in Cre levels at week 12 compared with the baseline (P < 0.05) (Table 3).
| Time | Hb in g/L | t | P value | Cre in mol/L | t | P value | CRP in mg/L | t | P value |
| Baseline | 114.82 ± 20.45 | 807.43 ± 254.64 | 8.60 ± 16.76 | ||||||
| Week 4 | 109.69 ± 19.78 | 1.530 | 0.131 | 749.67 ± 398.06 | 1.062 | 0.292 | 7.13 ± 10.71 | 0.642 | 0.523 |
| Week 12 | 111.47 ± 21.11 | 0.967 | 0.337 | 586.40 ± 358.51 | 4.326 | 0.000 | 7.72 ± 4.98 | 0.486 | 0.629 |
| Week 24 | 116.21 ± 23.50 | 0.380 | 0.705 | 803.27 ± 192.31 | 0.112 | 0.911 | 8.23 ± 14.82 | 0.206 | 0.838 |
During paricalcitol treatment, the Hb level was decreased in two cases (5.56%), and a transient elevation of serum P was found in one case (2.78%). After dose adjustment, all of these cases returned to normal.
The hemodialysis patients enrolled in this study also had SHPT and were treated with paricalcitol at a median starting dose of 0.06–0.08 µg/kg. iPTH levels decreased from 888.84 ± 376.88 to 598.88 ± 381.29 pg/mL after treatment. Twenty-one of 36 (58.33%) patients had a > 30% decrease in iPTH. The standard-reaching rate of iPTH (percentage of patients with iPTH levels down to 130–585 pg/mL) was 66.67% (24/36 patients). Koc et al[11] reported that after 6 mo of treatment, iPTH levels decreased from 518.9 to 264.0 pg/mL. There were 63.0% of patients with a > 30% decrease in iPTH levels. Olaizola et al[12] reported that after 6 mo of paricalcitol treatment in hemodialysis patients with SHPT, 17 of 19 (89.47%) patients had a > 30% decrease in iPTH levels. Twelve of 19 (63.16%) patients had iPTH levels down to 150–300 pg/mL. The effect of paricalcitol on iPTH levels was most significant in the first 4 wk. After that, iPTH levels changed less noticeably. This confirmed the efficacy of paricalcitol in inhibiting iPTH, which was coupled to progressive weakening of its inhibitory effect on iPTH over time. Therefore, excessive inhibition of iPTH caused by paricalcitol was prevented, which means that paricalcitol is safer than calcitriol.
Active vitamin D can stimulate intestinal Ca and P absorption by activating the intestinal VDRs, thereby contributing to hypercalcemia. Nonselective VDRAs, such as alfacalcidol and calcitriol, have no significantly different affinity for VDRs in intestinal mucosal cells and parathyroid cells. Paricalcitol is a highly selective VDRA with a higher affinity for VDRs in parathyroid cells than for those in intestinal mucosal cells. As intestinal Ca transport is weakened, the incidence of hypercalcemia decreases. Serum Ca and P levels in patients were detected in the present study. In the first 12 wk of paricalcitol treatment, there was a transient mild increase in average serum Ca levels. This has been reported in other studies[13] and may be considered a response in the adaptive period. Such a finding might have also been attributed to the diet of individual patients at the initial stage. Serum Ca levels stabilized after introduction of a Ca-restricted diet and the dose of paricalcitol was reduced. The Ca × P product decreased throughout the treatment period. After 24 wk of treatment, there were significant differences in serum P levels and Ca × P product compared with the baseline. These results indicated that paricalcitol reduced the risk of hyperphosphatemia. Li et al[14] reported no significant differences in the serum Ca and P levels and Ca × P product in hemodialysis patients with SHPT before and after paricalcitol treatment. Their findings disagree with ours, probably due to the differences in treatment duration.
The number and activity of osteoclasts usually increase in SHPT patients due to an excessively high iPTH level. Besides, bone transport and destruction are promoted, resulting in ALP elevation. In the present study, the ALP level decreased significantly after 24 wk of paricalcitol treatment compared with the baseline among the hemodialysis patients with SHPT, indicating that paricalcitol potentially corrects the SHPT-induced changes in bone histomorphology, which might be related to its inhibitory effect on bone metabolism. Some researchers believe that elevation of ALP is associated with a higher incidence of cardiovascular diseases in patients with chronic kidney disease. It is also one of the major reasons for the high mortality of hemo
The microinflammatory state in hemodialysis patients may be closely related to such complications as anemia and cardiovascular disease in hemodialysis patients. Some studies have shown that paricalcitol is not only effective for SHPT complicating hemodialysis but also benefits patients by regulating bone metabolism, participating in anti-inflammatory and antioxidative stress activities, and improving anemia[16]. Cre is the most common indicator of kidney function, and Hb is an important indicator of anemia. CRP not only indicates the inflammatory state but also participates in cardiovascular injury. It has been found that during paricalcitol treatment, Hb and CRP levels at different time points are not significantly different from those at the baseline. In our study, at week 12, the Cre level was markedly reduced compared with the baseline. Later, the Cre level began to increase. These changes suggested that paricalcitol had no evident effect on kidney function indicators and inflammatory factors while reducing iPTH levels. The fact that the Cre level first decreased and then increased might be explained by the abnormal kidney function in hemodialysis patients. Paricalcitol may reduce the release of inflammatory factors such as CRP[17]. It is reported that paricalcitol has no significant impact on the inflammatory factors in hemodialysis patients with SHPT[18], which agrees with our findings.
In conclusion, paricalcitol significantly decreased serum levels of iPTH, ALP and P in hemodialysis patients with SHPT. In contrast, serum Ca, Hb, Cre and CRP levels remained stable. However, our study had a small sample size without a control group. In future, multicenter studies with a larger sample size will be performed to provide evidence for the clinical use of paricalcitol.
Secondary hyperparathyroidism (SHPT) is a common complication in patients with end-stage renal disease. SHPT is a component of chronic kidney disease-mineral and bone disorder, which is featured by increased fibroblast growth factor 23 and serum parathyroid hormone concentrations, decreased 1,25(OH)2 vitamin D concentrations and abnormal serum phosphate and calcium concentrations.
The long-term use of vitamin D receptor activators (VDRAs) may enhance the intestinal absorption of calcium and phosphorus and tubular reabsorption, leading to an increase in serum levels of calcium and phosphorus and risk of vascular calcification. But Paricalcitol mildly affects intestinal calcium and phosphorus absorption. Paricalcitol may be better than VDRAs in this aspect.
This study aimed to discuss the outcome, safety and other potential benefits of paricalcitol injection in hemodialysis patients with SHPT.
Total 40 patients who received hemodialysis for chronic renal failure with SHPT received paricalcitol injection for 24 wk, three times per week. The primary outcome indicator was the percentage of patients with a > 30% decrease in intact parathyroid hormone (iPTH) levels at week 24 compared with the baseline.
After 24 wk of treatment, iPTH levels decreased significantly. More than 30% decrease of iPTH was found in 21 of 36 (58.33%) patients. The average decrease in iPTH levels was 32.16 ± 4.33%; the standard-reaching rate of iPTH levels was 66.67% (24/36); and alkaline phosphatase levels decreased significantly compared with the baseline. There were no significant differences in the serum levels of calcium, hemoglobin, creatinine and C-reactive protein compared with the baseline.
This study suggested that the paricalcitol was a safe and effective treatment for hemodialysis patients with SHPT.
Multicenter studies with a larger sample size will be performed to provide evidence for the clinical use of paricalcitol.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Urology and Nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Takahashi T S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Messa P, Alfieri CM. Secondary and Tertiary Hyperparathyroidism. Front Horm Res. 2019;51:91-108. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7:1-59. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008;74:276-288. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-38. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Mizobuchi M, Ogata H, Koiwa F. Secondary Hyperparathyroidism: Pathogenesis and Latest Treatment. Ther Apher Dial. 2019;23:309-318. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Cocchiara G, Fazzotta S, Palumbo VD, Damiano G, Cajozzo M, Maione C, Buscemi S, Spinelli G, Ficarella S, Maffongelli A, Caternicchia F, Ignazio Lo Monte A, Buscemi G. The medical and surgical treatment in secondary and tertiary hyperparathyroidism. Review. Clin Ter. 2017;168:e158-e167. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Tanaka M, Tokunaga K, Komaba H, Itoh K, Matsushita K, Watanabe H, Kadowaki D, Maruyama T, Otagiri M, Fukagawa M. Vitamin D receptor activator reduces oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2011;15:161-168. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Brancaccio D, Bommer J, Coyne D. Vitamin D receptor activator selectivity in the treatment of secondary hyperparathyroidism: understanding the differences among therapies. Drugs. 2007;67:1981-1998. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Zhang T, Ju H, Chen H, Wen W. Comparison of Paricalcitol and Calcitriol in Dialysis Patients with Secondary Hyperparathyroidism: A Meta-Analysis of Randomized Controlled Studies. Ther Apher Dial. 2019;23:73-79. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] [Cited in This Article: ] |
| 11. | Koc H, Hoser H, Akdag Y, Kendir C, Ersoy FF. Treatment of secondary hyperparathyroidism with paricalcitol in patients with end-stage renal disease undergoing hemodialysis in Turkey: an observational study. Int Urol Nephrol. 2019;51:1261-1270. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Olaizola I, Caorsi H, Fajardo L, Ferreiro A, Campistrus N, Dolinsky D, Petraglia A, Ambrosoni P. Effectiveness and safety of a 6-month treatment with paricalcitol in patients on hemodialysis with secondary hyperparathyroidism. J Bras Nefrol. 2016;38:302-312. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Wang Q, Chang L, Li Y, Li T, Zhang L, Zhou Y. [Application of paricalcitol in the treatment of patients with maintenance hemodialysis]. Xiandai Shengwuyixue Jinzhan. 2018;18:3459-3462, 3596. [DOI] [Cited in This Article: ] |
| 14. | Li J, Li X, Wang Y. [Efficacy and safety of paricalcitol on secondary hyperparathyroidism in hemodialysis patients]. Zhongguo Xueye Jinghua. 2019;18:386-389. [DOI] [Cited in This Article: ] |
| 15. | Wang TN, Xu B, Jia FY, Zhang HT, Gong DH, Liu ZH. [Treatment of secondary hyperparathyroidism in hemodilysis patients by paricalcitol]. Shenzangbing Yu Touxi Shenyizhi Zazhi. 2015;24:1-5. [Cited in This Article: ] |
| 16. | Zhang XQ, Lu C. [Progress in the treatment of secondary hyperparathyroidism with paricalcitol and calcitriol]. Linchuang Shenzangbing Zazhi. 2017;17:761-764. [DOI] [Cited in This Article: ] |
| 17. | Navarro-González JF, Donate-Correa J, Méndez ML, de Fuentes MM, García-Pérez J, Mora-Fernández C. Anti-inflammatory profile of paricalcitol in hemodialysis patients: a prospective, open-label, pilot study. J Clin Pharmacol. 2013;53:421-426. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Yun Y, Zhang C, Liu NQ, Zhou GY, LI DT. [The effects of paricalcitol on inflammatory state and oxidative stress in maintenance hemodialysis patients]. Zhongguo Xueye Jinghua. 2018;17:677-681. [Cited in This Article: ] |