Published online Apr 6, 2020. doi: 10.12998/wjcc.v8.i7.1306
Peer-review started: November 11, 2019
First decision: January 19, 2020
Revised: February 26, 2020
Accepted: February 28, 2020
Article in press: February 28, 2020
Published online: April 6, 2020
Neurofibromatosis (NF) is a genetic disease consisting of seven types, of which types 1 to 4 are caused by a dominant autosomal gene mutation; such disease sometimes arises in patients with NF type 1. However, it remains unclear whether the origin of neurofibrosarcoma is directly linked to the incidence of NF type 1, as no reports have been published on this issue. Here, we report a case of NF1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
A 51-year-old male was admitted to our hospital due to fever accompanied by coughing, chest tightness and asthma for more than one month. The preliminary diagnosis was NF type 1, which was pathologically confirmed by a subsequent thoracoabdominal subcutaneous biopsy. The definitive diagnosis was neurofibrosarcoma with a pathogenic NF1 gene. The patient refused surgery and chemoradiotherapy, and died two months later. NF is a genetic disease consisting of seven types, of which types 1 to 4 are caused by a dominant autosomal gene mutation. The case reported belongs to the class of NF1-positive dominant inheritance. Neurofibrosarcoma is a malignant tumor derived from cells surrounding the peripheral nerves. However, due to the lack of previous reports, it remains unclear whether the origin of neurofibrosarcoma is directly linked to the incidence of NF type 1.
We report the first case of NF1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
Core tip: Neurofibromatosis is a genetic disease with 7 types, of which type 1 to 4 are caused by dominant gene mutation on the autosome. Neurofibrosarcoma is a malignant tumor derived from cells surrounding the peripheral nerves, usually found in the limbs but seldom metastasizing to other parts of the body, although it may reach the lungs by extensive spread along nerve tissues. It mostly affects young and middle-age adults, and sometimes arises in patients with neurofibromatosis type 1. We herein, report the first case of neurofibromatosis type 1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
- Citation: Wang Y, Lu XF, Chen LL, Zhang YW, Zhang B. Multiple neurofibromas plus fibrosarcoma with familial NF1 pathogenicity: A case report. World J Clin Cases 2020; 8(7): 1306-1310
- URL: https://www.wjgnet.com/2307-8960/full/v8/i7/1306.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i7.1306
Neurofibromatosis (NF) is a genetic disease consisting of seven types, of which types 1 to 4 are caused by a dominant autosomal gene mutation[1,2]. Neurofibrosarcoma is a malignant tumor derived from cells surrounding the peripheral nerves and usually occurs in the limbs. Neurofibrosarcoma seldom metastasizes to other parts of the body, although it may reach the lungs by extensive spread along nerve tissues[3]. This disease predominantly affects young and middle-age adults, and sometimes arises in patients with NF type 1. However, it remains unclear whether the origin of neurofibrosarcoma is directly linked to the incidence of NF type 1, as no reports have been published on this issue. Here, we report a case of NF1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
The patient was admitted to our hospital due to cough and sputum with intermittent fever for more than one month.
The patient had no obvious cause of cough and excessive phlegm before one month. The sputum was white and easy to cough; the cough was not related to body position, activity, etc. Fever and chills were not observed.
Normal health was fair. No history of chronic diseases such as hypertension, diabetes, or coronary heart disease, but he had a history of scoliosis for decades, and a history of multiple lipomas for many years.
No clear family history.
Temperature was 36.9°C, pulse rate was 133 beats/min, respiratory rate was 25 breaths/min, blood pressure was 119/87 mmHg, and body mass index was 24 kg/m2. Pigmentation was visible throughout the body with multiple lipomas.
White blood cell count was 12.29 × 109/L, NE% was 89.21%, hemoglobin was 151 g/L, platelet count was 296 × 109/L, and no obvious abnormalities were noted in liver and kidney function. C-reactive protein was 69.4 mg/L, thyroid-stimulating hormone was 5.17 uIU/mL, and both triiodothyronine and thyroxine were normal.
Chest computed tomography (CT) revealed occupancy in the right lower lobe and right hilar, right lower lobar inflammation with consolidation, and right pleural effusion; color Doppler ultrasound revealed solid right adrenal gland occupancy with metastatic potential, left cervical lymph node swelling; no malignant tumor cells were found in the biopsy, and no obvious enlarged lymph nodes on the bilateral clavicle.
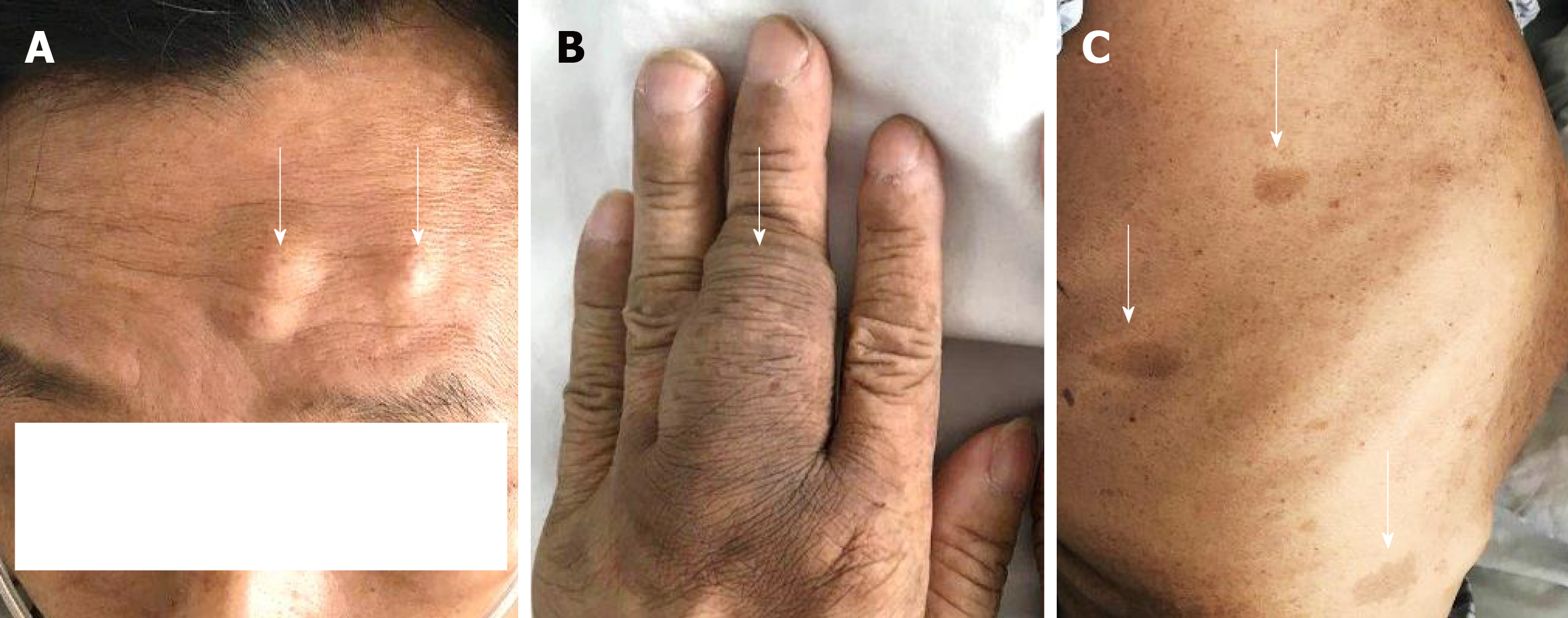
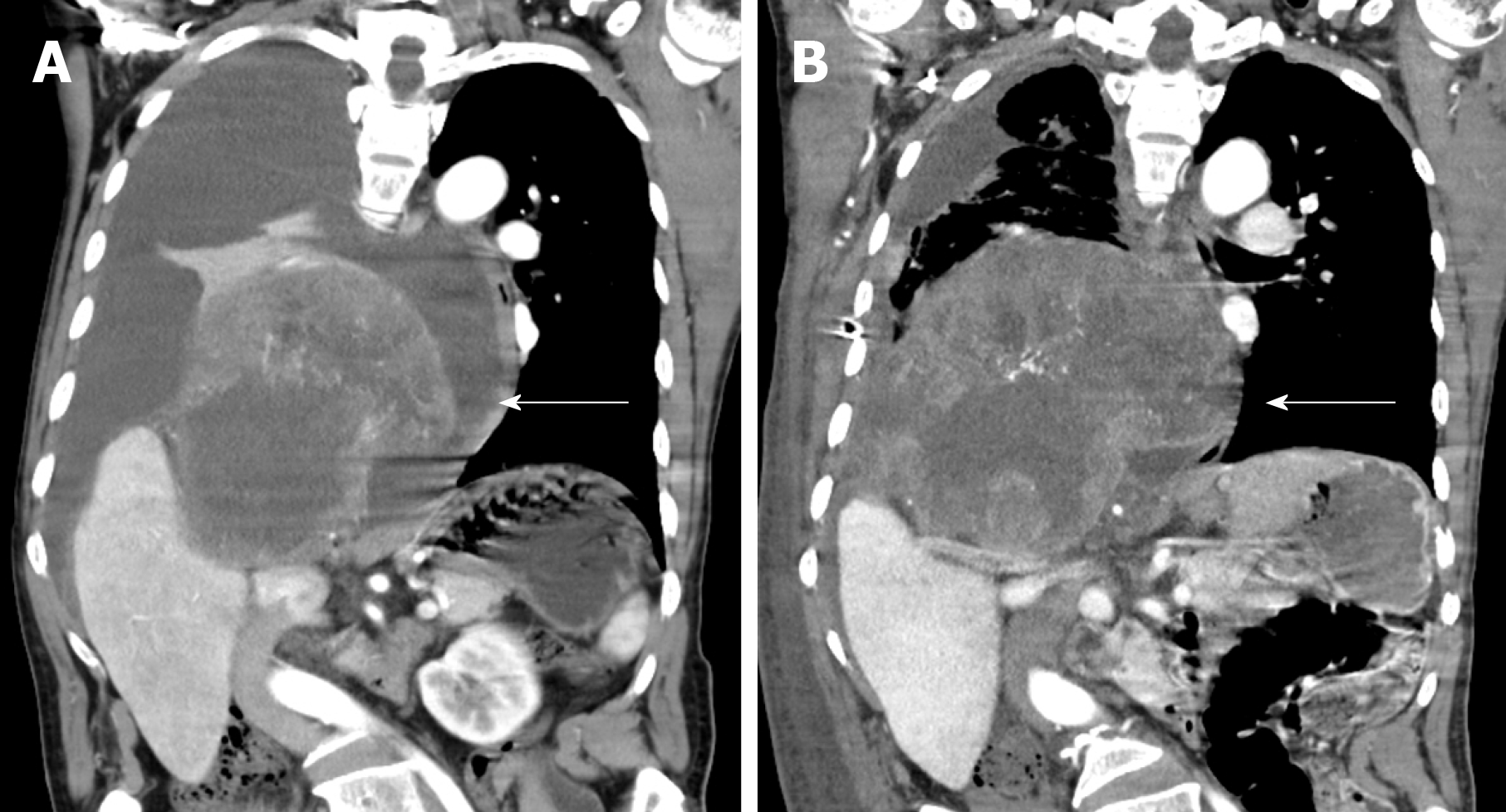
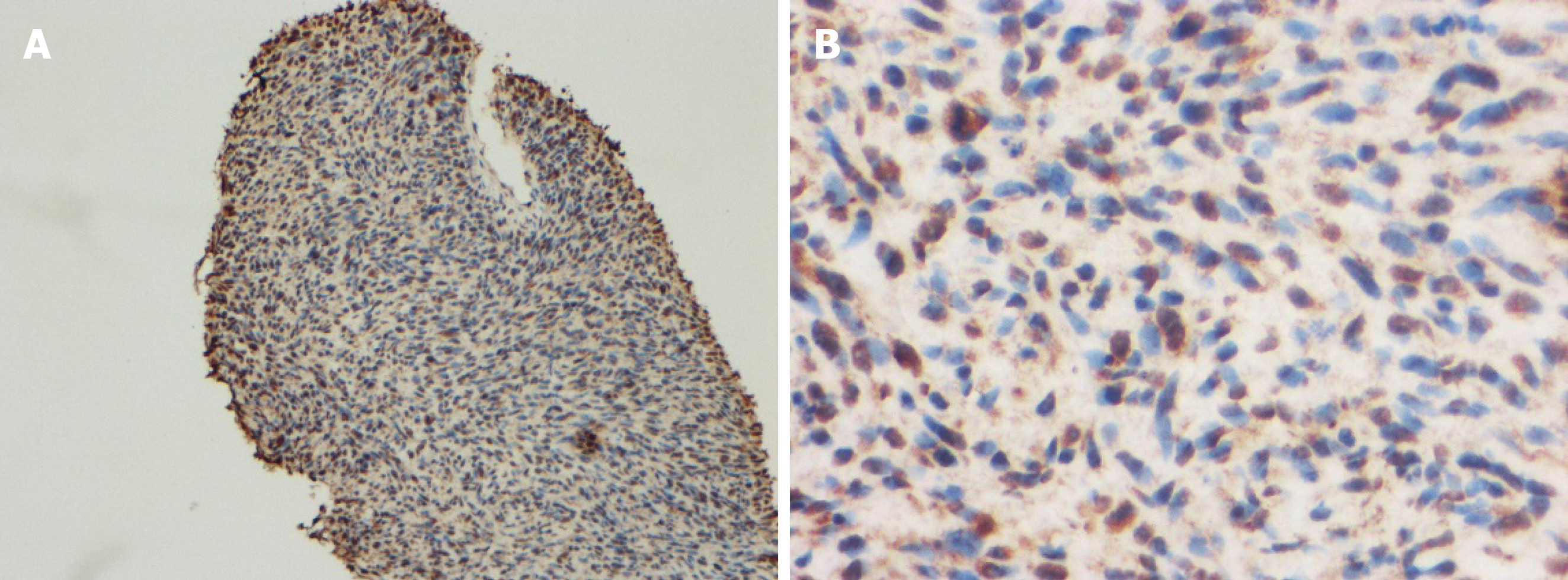
The 51-year-old male was admitted to our hospital in January 2018 with fever accompanied by coughing, chest tightness and asthma for more than one month. Multiple subcutaneous nodules, which were soft and mobile, were observed in the forehead and limbs (Figure 1A and 1B). Multiple areas of pigmentation were seen throughout the body such as the right hypochondrium of the torso (Figure 1C). The spine showed curvature to the right side, and thoracoabdominal contrast-enhanced CT imaging revealed a large mass in the right thoracic cavity, accompanied by atelectasis and extensive hydrothorax on the right side (Figure 2). No malignant cells were observed in the hydrothorax smear and the hydrothorax chylous test was positive. Our examination indicated a high possibility of lymphangiomatosis and right pleural space occupation was initially suspected. To further confirm the diagnosis of chylothorax, triglyceride and cholesterol tests were performed on the pleural fluid. The results revealed a low level of triglycerides and a cholesterol-to-triglyceride ratio exceeding 1 in the pleural fluid, which suggested a diagnosis of pseudochylothorax. The preliminary diagnosis was NF type 1, which was pathologically confirmed by a subsequent thoracoabdominal subcutaneous biopsy. B-ultrasound-guided percutaneous lung puncture was performed to clarify the nature of the pleural space occupation, and the pathological results indicated a spindle cell malignancy suggestive of a high-grade sarcoma. The definitive diagnosis was neurofibrosarcoma (Figure 3). To determine the existence of a familial genetic factor, the NF1 gene was sequenced from peripheral blood samples from the patient and his daughter. The results showed that both carried the pathogenic gene of NF1 (chr17q11 | NM_000267.3) with the c1885G>A mutation located in Exon 17. The patient refused surgery and chemoradiotherapy, and died two months later.
The final diagnosis in this patient was NF1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
The patient refused surgery and chemo-radiotherapy.
The patient died two months later.
This patient was classified as a case of NF1-positive dominant inheritance. NF type 1 rarely involves internal organs, although histological evidence of disease in this case was found in the mediastinum. The majority of neurofibromas in the mediastinum originate from the spinal or intercostal nerves and are located in the posterior mediastinum[4], while the tumor in this case was in the middle mediastinum. This may be associated with the anatomical position of the vagus nerve. Moreover, although NF tends to be benign, in this case, a malignant sarcomatoid tumor was accompanied by ipsilateral pleural effusion, which is rarely encountered. Based on our initial examinations, we made a diagnosis of chylothorax caused by lymphangiomatosis. Lymphangioma is a rare tumor that originates in the lymphatic system, and may develop in any region of the body through which lymphatic vessels pass[5]. On CT imaging, a cystic mass in the mediastinum often appears as a homogeneous low-density area[6,7], although other forms of the disease may be present if the tissue composition of the mass varies[8,9]. In the case described here, preliminary examinations showed that the patient’s pleural fluid was turbid, bloody and chylomicron-positive, which, in combination with the large mass in the pleural cavity, suggested chylothorax. However, further examinations led to the diagnosis of pseudochylothorax. Pseudochylothorax is a type of pleural effusion that is less common than chylothorax, and is predominantly caused by cholesterol accumulation[10,11]. In the case of pseudochylothorax, the proportion of cholesterol-to-triglyceride in the fluid always exceeds 1, with cholesterol > 200 mg/dL and triglyceride < 110 mg/dL.
We report the first case of NF1-positive multiple neurofibromas with malignant fibrosarcomatous transformation in the pleural cavity.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cuocolo R, El-Razek AA S-Editor: Wang J L-Editor: Webster JR E-Editor: Xing YX
| 1. | Cunha KS, Barboza EP, Dias EP, Oliveira FM. Neurofibromatosis type I with periodontal manifestation. A case report and literature review. Br Dent J. 2004;196:457-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Razek AAKA. MR imaging of neoplastic and non-neoplastic lesions of the brain and spine in neurofibromatosis type I. Neurol Sci. 2018;39:821-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Guo A, Liu A, Wei L, Song X. Malignant peripheral nerve sheath tumors: differentiation patterns and immunohistochemical features - a mini-review and our new findings. J Cancer. 2012;3:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Reeder LB. Neurogenic tumors of the mediastinum. Semin Thorac Cardiovasc Surg. 2000;12:261-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zhuang K, Jiang X, Huang S. A Rare, Giant, Cystic, and Cavernous Lymphangioma Originated from the Stomach in a Young Woman. J Gastrointest Surg. 2019;23:1271–1273. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Charruau L, Parrens M, Jougon J, Montaudon M, Blachère H, Latrabe V, Laurent F. Mediastinal lymphangioma in adults: CT and MR imaging features. Eur Radiol. 2000;10:1310-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Surov A, Nagata S, Razek AA, Tirumani SH, Wienke A, Kahn T. Comparison of ADC values in different malignancies of the skeletal musculature: a multicentric analysis. Skeletal Radiol. 2015;44:995-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Restrepo CS, Pandit M, Rojas IC, Villamil MA, Gordillo H, Lemos D, Mastrogiovanni L, Diethelm L. Imaging findings of expansile lesions of the thymus. Curr Probl Diagn Radiol. 2005;34:22-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Razek AAKA, Ashmalla GA. Assessment of paraspinal neurogenic tumors with diffusion-weighted MR imaging. Eur Spine J. 2018;27:841-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 10. | Villena Garrido V, Ferrer Sancho J, Hernández Blasco L, de Pablo Gafas A, Pérez Rodríguez E, Rodríguez Panadero F, Romero Candeira S, Salvatierra Velázquez A, Valdés Cuadrado L; Area de Tecnicas y Trasplantes. SEPAR. [Diagnosis and treatment of pleural effusion]. Arch Bronconeumol. 2006;42:349-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Hirano T, Fujino N, Takase K, Ota H, Tanaka R, Saito R, Suzuki A, Okutomo K, Sato T, Kageyama S, Tamada T, Sugiura H, Ichinose M. Pulmonary Lymphatic Perfusion Syndrome. Am J Respir Crit Care Med. 2019;199:529-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |