Published online Feb 26, 2020. doi: 10.12998/wjcc.v8.i4.838
Peer-review started: December 14, 2019
First decision: December 30, 2019
Revised: January 1, 2020
Accepted: January 11, 2020
Article in press: January 11, 2020
Published online: February 26, 2020
Secondary malignancy of the thyroid occurs infrequently and mainly originates from malignant tumors of the kidney, gastrointestinal tract, lungs, breast, and skin. The correct diagnosis is important but difficult. Importantly, there are major differences in the treatment of primary and metastatic thyroid cancer, which has a significant impact on prognosis and survival. Therefore, how to diagnose thyroid metastasis (TM) correctly before surgery is a major concern for surgeons.
We report a 38-year-old woman who presented with palpable cervical lymph nodes after breast cancer (BC) surgery 2 years ago. Ultrasonography and computed tomography revealed thyroid nodules with irregular margins and enlarged cervical lymph nodes. Biopsy was performed for the right largest cervical lymph node, and immunohistochemical analysis revealed negativity for thyroglobulin, estrogen receptor, and progestin receptor and positive for human epidermal growth factor receptor 2. The diagnosis was TM from BC with cervical lymph node metastasis. Total thyroidectomy with bilateral central and lateral neck lymph node dissection was performed. After a 5-mo follow-up, no recurrence or novel distant metastasis was identified.
TM from BC is a rare secondary malignancy. Broad differential diagnosis by biopsy and immunohistochemical analysis needs to be considered.
Core tip: Thyroid metastasis from breast cancer is a rare secondary malignancy. The correct diagnosis before surgery is important but difficult. Importantly, there are major differences in the treatment of primary and metastatic thyroid cancer, which has a significant impact on prognosis and survival. Because of its low incidence, thyroid metastasis from breast cancer is easily overlooked and misdiagnosed. Broad differential diagnosis by biopsy and immunohistochemical analysis needs to be considered.
- Citation: Zhang YY, Xue S, Wang ZM, Jin MS, Chen ZP, Chen G, Zhang Q. Thyroid metastasis from breast cancer presenting with enlarged lateral cervical lymph nodes: A case report. World J Clin Cases 2020; 8(4): 838-847
- URL: https://www.wjgnet.com/2307-8960/full/v8/i4/838.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i4.838
Although the thyroid gland is highly vascularized, secondary malignancy, which metastasizes to the thyroid, occurs infrequently, accounting for only 1%-3% of thyroid cancers[1,2]. However, reports suggest that thyroid metastases (TM) are possibly more common than primary thyroid carcinoma (TC) by autopsy series[3]. Usually, secondary malignancy of the thyroid gland originates from renal cell carcinoma, followed by malignant tumors of the gastrointestinal tract, lungs, breast, and skin[4,5]. More importantly, these cases are diagnostically challenging. There are major differences in the treatment of primary and metastatic thyroid cancer, which has a significant impact on prognosis and survival. Therefore, determining a correct diagnosis before surgery is a major concern for surgeons. In addition, patients with metastatic breast cancer (BC) to the thyroid usually present with symptoms of a new or enlarging thyroid nodule[6]. TM from BC presenting with enlarged lateral cervical lymph nodes is rare. Here, we report a case of bilateral thyroid and bilateral cervical lymph node metastasis from left invasive ductal carcinoma along with a brief literature review of similar cases. A literature search was performed with key words (thyroid cancer, breast cancer, and metastasis) for studies regarding TM from BC until December 2019.
A 38-year-old woman was admitted to our clinic in July 2019 for bilateral cervical palpable lymph nodes.
The patient was euthyroid, and there were no high metabolic symptoms, such as heat, sweating, and hand shaking, during the course of the disease. Biopsy was carried out in another hospital for the right enlarged cervical lymph node. Immunohistochemical analysis revealed negativity for thyroglobulin (TG), estrogen receptor (ER), and progesterone receptor (PR) and positive for human epidermal growth factor receptor 2 (Her-2). There was no obvious bone metastasis in the whole-body bone scan.
In March 2017, she was diagnosed with left breast invasive ductal carcinoma with Paget's disease and treated by mammectomy and axillary lymph node dissection followed by chemotherapy and radiotherapy. The patient underwent chemotherapy (docetaxel and capecitabine) and Herceptin targeted therapy for another 12 mo.
The patient had no significant personal or family history.
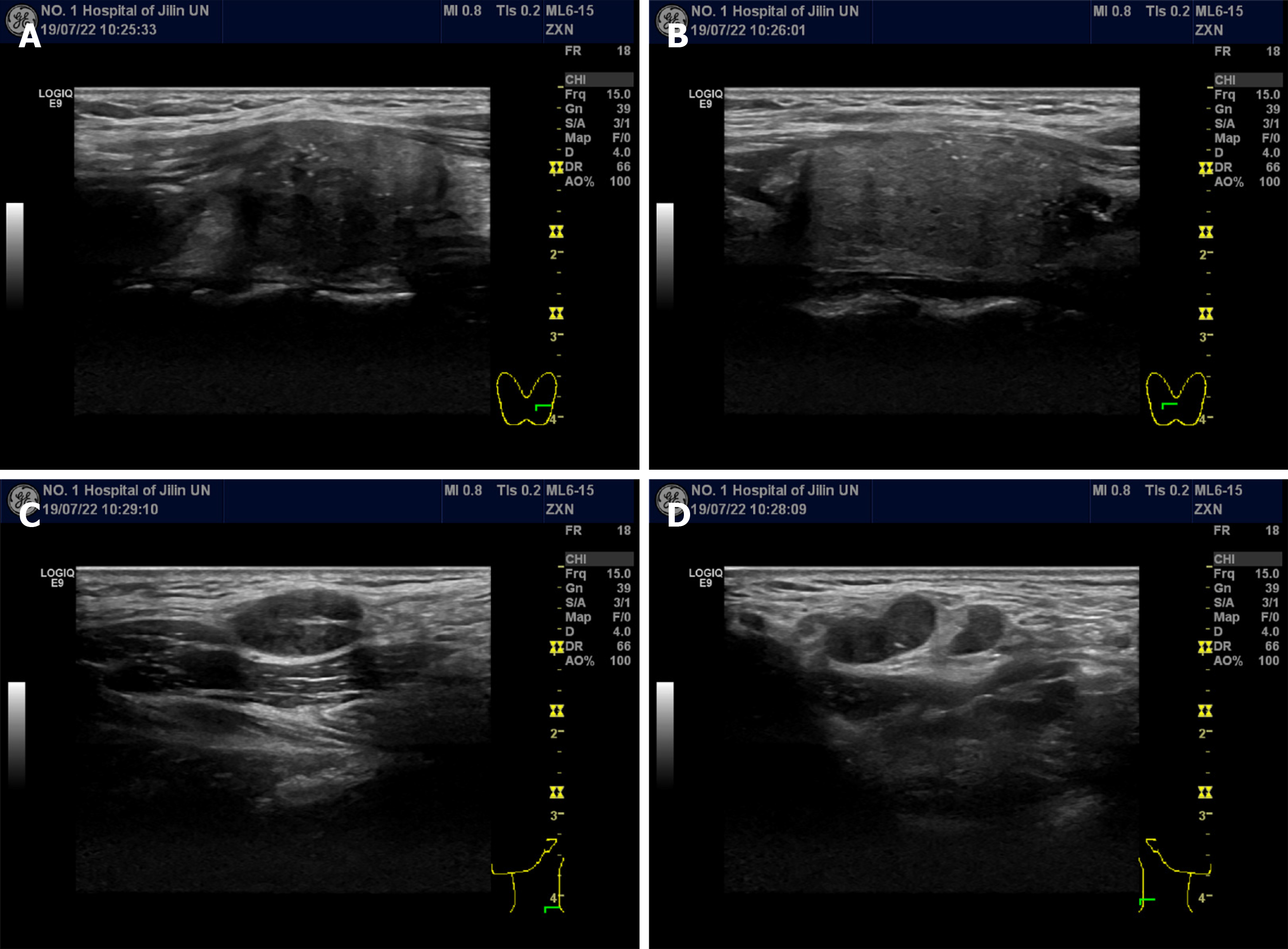
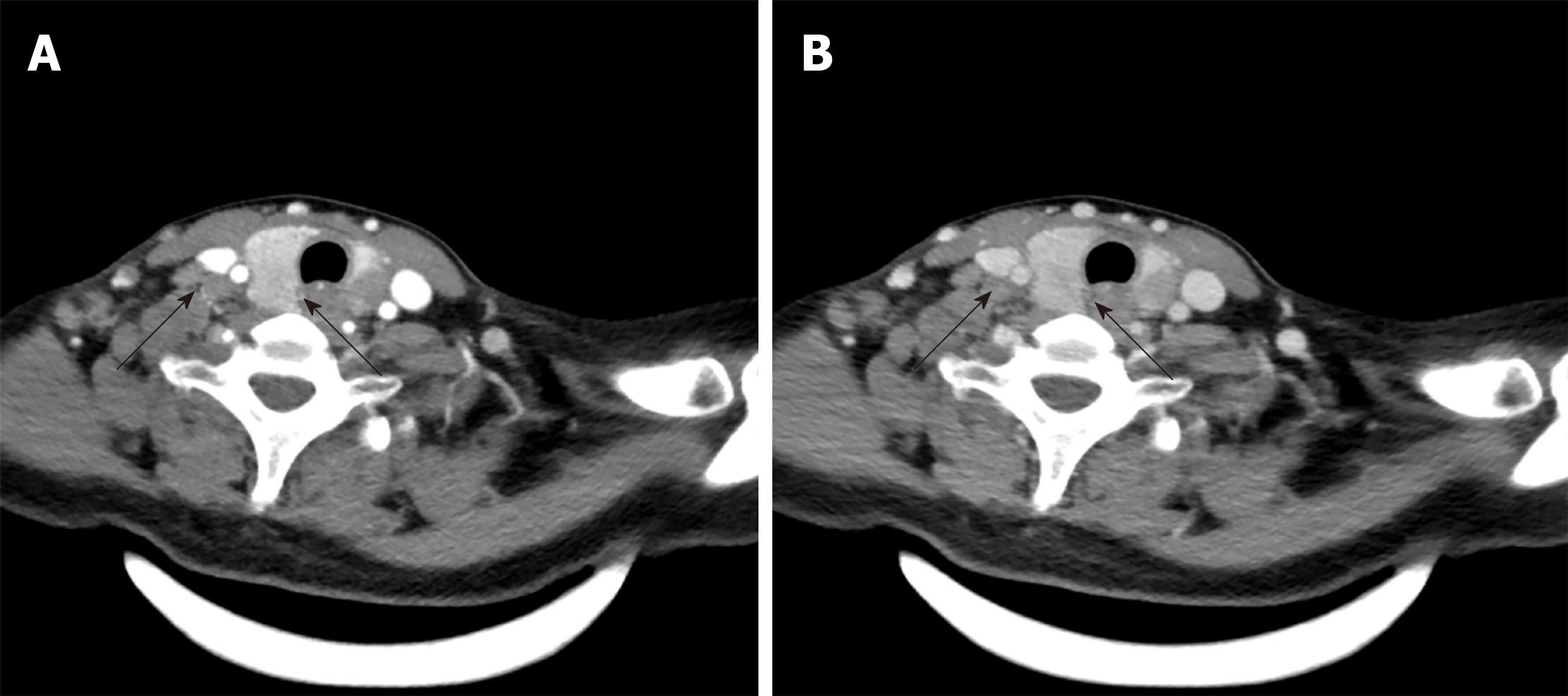
Ultrasonography (US) revealed solid, hypoechoic thyroid nodules with irregular margins and multiple enlarged lymph nodes (Figure 1). Computed tomography (CT) showed multiple bilateral lymph nodes with diameters ranging from 0.4 cm to 1.2 cm. Contrast-enhanced CT showed homogenous enhancement (Figure 2).
TM from BC with cervical lymph node metastasis.
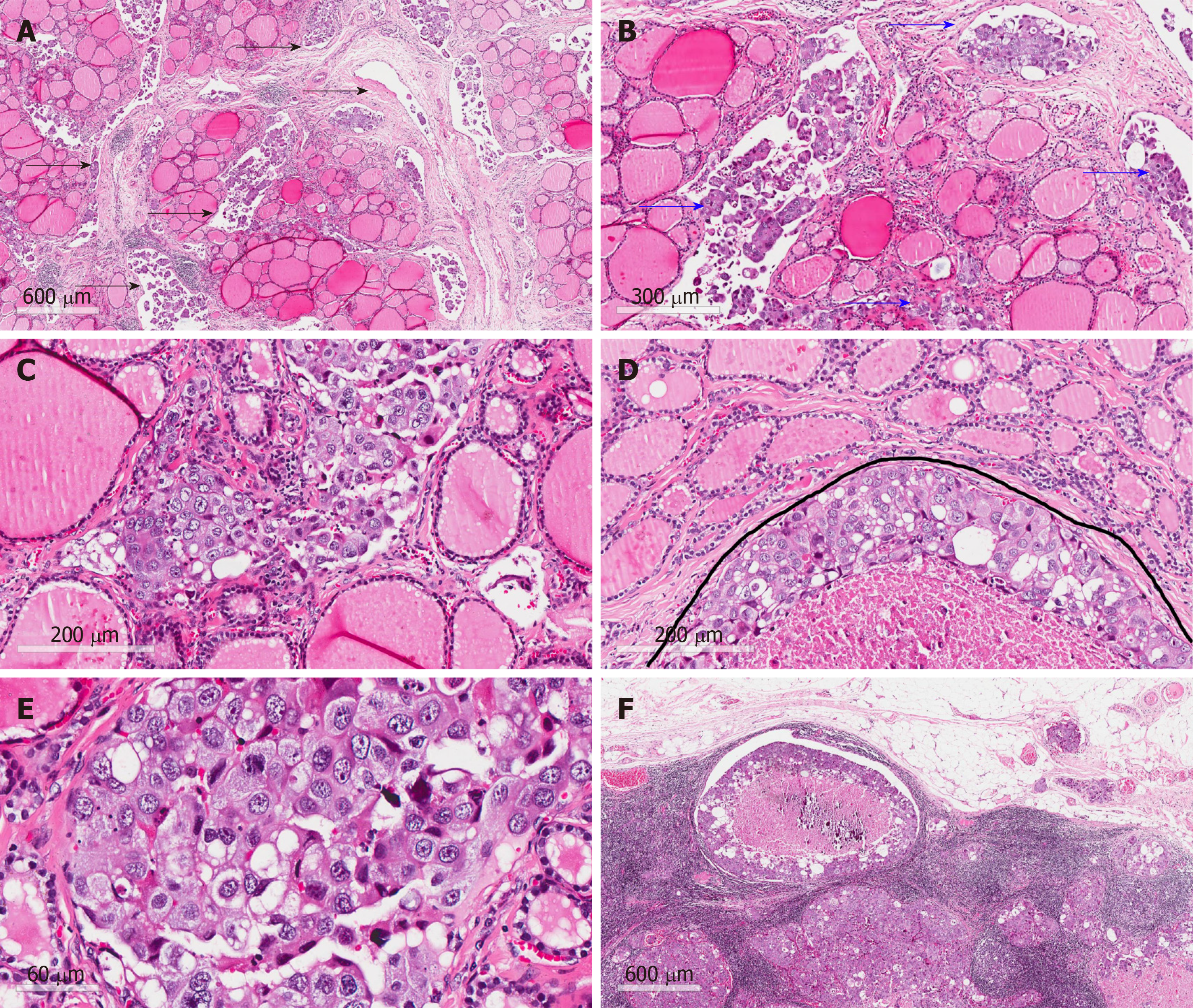
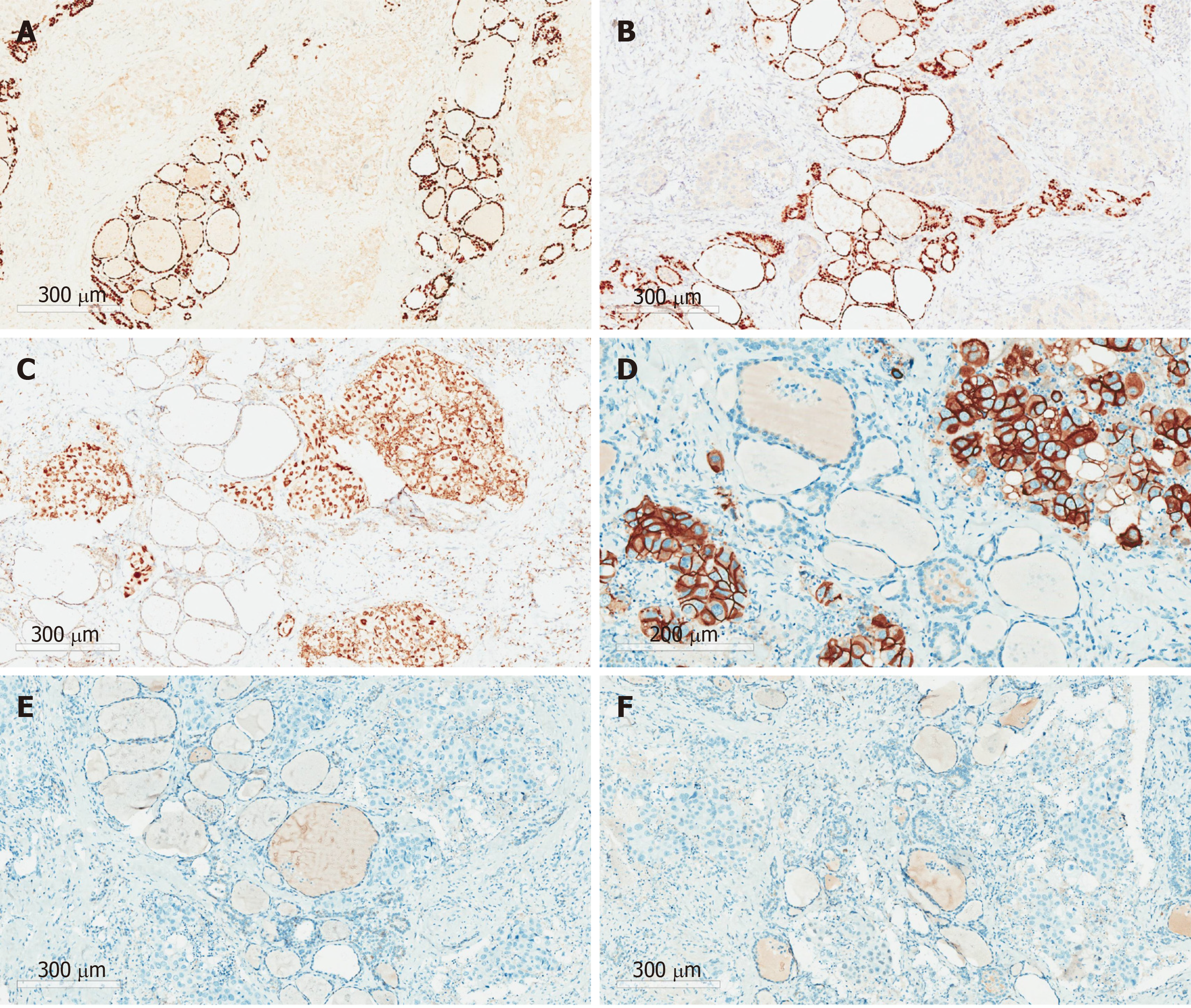
Total thyroidectomy and bilateral central and lateral neck lymph node dissection were performed for this patient. Postoperative pathology showed extensive intravascular infiltration and nerve infiltration of breast invasive ductal carcinoma in the bilateral thyroid tissue, accompanied by interstitial fibrous tissue hyperplasia, scattered necrosis, and calcification (Figure 3). The positive status of cervical lymph nodes was: Central lymph nodes (3/3), the left lateral neck from level II-IV lymph nodes (7/12), and the right lateral neck from level II-IV lymph nodes (14/14). The results of immunohistochemistry indicated the following: ER (-), Her-2 (3+), PR (-), GATA3 (+), PAX-8 (-), and TTF-1 (-) (Figure 4).
One month after surgery, the patient was admitted to the Oncology Department of our hospital for postoperative examination. Breast ultrasound examination was performed, indicating that the right breast mass was BI-RADS 3. Currently, no recurrence or novel distant metastasis was identified after a 5-mo follow-up.
TM is uncommon, and breast-derived TM is relatively rare. We searched PubMed until December 2019 and identified 15 reports about metastases to the thyroid from BC (Table 1)[1,4,6-18]. According to this literature review and analysis, some preliminary conclusions can be made as follows: (1) The occurrence of TC may have no correlation with age, and all reported cases were female; (2) among BC, invasive ductal carcinoma is a more common histopathological type that metastasizes to the thyroid than others, such as invasive lobular carcinoma and metaplastic breast carcinoma; (3) the interval time from BC diagnosis to TC ranges from 6 months to 288 mo. Therefore, for patients with thyroid nodules or cervical lymph nodes who have a history of malignant breast tumors, regardless of the course of the disease, the possibility of metastatic cancer should be considered; and (4) preoperative biopsy and immunohistochemical analysis can assist in the diagnosis of TM and primary malignancy.
| Ref. | No of cases | Age (yr) | Type of BC | Location of BC | Location of TC | Time for metastasis (mo) | Metastases elsewhere | Immunohistochemistry | Treatment |
| Inic et al[1] | 1 | 28 | Lobular | Right | Bilateral | 60 | Lymph nodes in right lateral neck | Mammaglobin (+), GCDFP-15 (+), ER (+), PR (+), Tg (-), TTF-1 (-), CK7 (-) HER2 (-) | TT, right CLND and LLND |
| Liu et al[4] | 1 | 47 | Invasive ductal | Bilateral | Right | 48 | Bilateral cervical lymph nodes | ER (+), PR (+), HER2 (+) | Apatinib |
| Gong et al[6] | 1 | 57 | Metaplastic | Right | Left | 24 | NA | NA | NA |
| Zhou et al[7] | 8 | 55.37 | NA | Right (n = 5); left (n = 1); bilateral (n = 2) | NA | 6 to 121 | Chest wall (n = 2); supraclavicular nodes (n = 2); lung (n = 2); axilla nodes (n = 2); cervical and mediastinal lymph node (n = 2) | TG (-) and TTF-1 (-) | TT or lobectomy or chemotherapy |
| Ghias et al[8] | 1 | 67 | Invasive ductal | NA | Bilateral | 24 | Brain | AE1/AE3 (+), ER (+), GATA3 (+), E-cadherin (+), CK7 (-), CK20 (-), GCDFP/mammaglobin (-), TTF-1 (-), Tg (-), CT (-), synaptophysin (-) | Lobectomy |
| Yang et al[9] | 1 | 51 | Invasive ductal | Left | Left | 288 | Cervical lymph nodes | ER (+), PR (-) | TT with a selective LLND and postoperative chemotherapy |
| Owens et al[10] | 1 | 64 | Infiltrating mammary | NA | Left | 60 | Subcutaneous mass in the right shoulder and liver | HER2 (+) | No surgery |
| Magers et al[11] | 1 | 37 | Ductal breast | NA | Right lobe and isthmus | 72 | Bone and brain | GATA-3 (+), ER (+), PAX-8 (-), TTF-1 (-) | NA |
| Durmo et al[12] | 1 | 72 | Invasive ductal | NA | Left | NA | The third lumbar vertebrae and left iliac bone | NA | NA |
| Yang et al[13] | 1 | 45 | Infiltrating ductal | Right | Bilateral | 36 | Bilateral cervical lymph nodes | ER (+), PR (+), CerbB-2 (+), TTF-1 (-), Tg (-) | Bilateral subtotal thyroidectomy and lymphadenectomy |
| Zeng et al[14] | 1 | 49 | Inflammatory | Right | NA | 7 | Lymph nodes in right lateral neck | CA15-3 (+), Her2 (+++), GCDFP-15 (-), TG (-), TTF-1 (-), ER (-), PR (-) | TT, CLND, LLND and postoperative RAI and chemotherapy |
| Lacka et al[15] | 1 | 54 | Ductal-lobular mammary | Left | Bilateral | 168 | Skeletal supraclavicular lymph nodes, liver, and right lung | CKMNF 116 (+), CK7 (++), CEA (+), MGB2 (++), ER (+), PR (+), HER2 (+), CK20 (-), TG (-), TTF1 (-), CT (-), chromogranin (-) | TT and bilateral CLND and LLND with chemotherapy |
| Bourcier et al[16] | 1 | 54 | Invasive lobular | NA | left | 0 | Left supraclavicular and cervical lymph nodes | TG (-), TTF-1 (-), E-cadherin (-), HER-2 (-), AE1/AE3(+) ER (+), PR (+), CDX2 (+), PAX8 (+), GATA3(+) | TT and CLND |
| Pensabene et al[17] | 1 | 64 | Invasive lobular | Left | left | 18 | Bone | Cytokeratin-19 (+) ER (+) tireoglobulin (-), e-cadherin (-), cytokeratin-7 (-) | Lobectomy |
| Plonczak et al[18] | 1 | 62 | Invasive ductal | Bilateral | NA | 144 | Lung and bone, both lateral cervical lymph nodes | CEA (+), GATA3 (+), GCDFP-15 (+), CK7 (+), TG (-), TTF-1 (-), CK20 (-), PR (-), HER-2 (-) | TT and CLND |
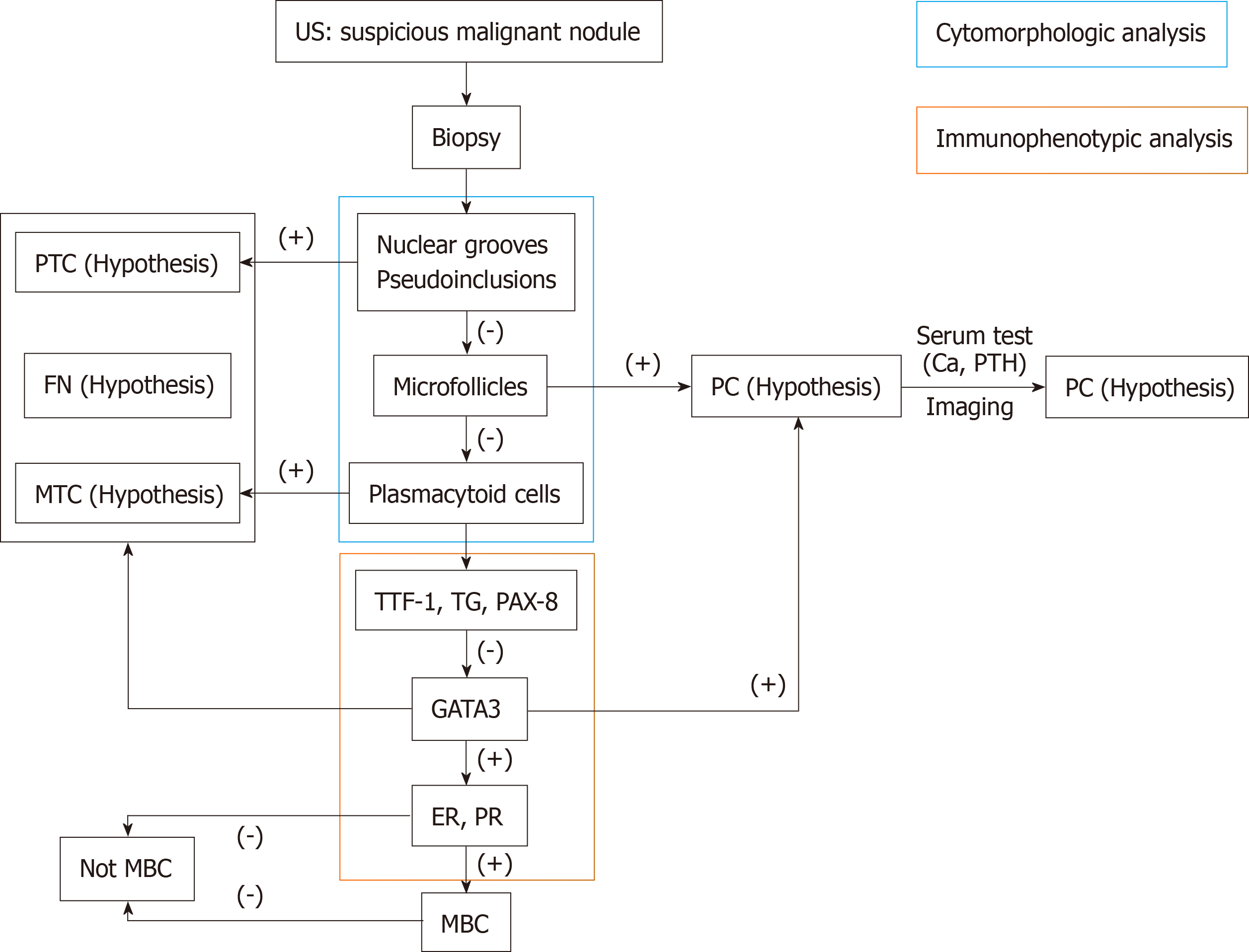
TM has no difference in symptoms from primary thyroid cancer, and the physical examination is usually normal. TMs from renal cell carcinoma are usually symptomatic, such as neck masses, dysphagia, and hoarseness, while lung cancer and BC usually have no symptoms and are mostly found by US[19]. In this case, the patient with TM from BC initially presented with enlarged cervical lymph nodes, which has not been reported before to our knowledge. Therefore, preoperative diagnosis of TM from BC is difficult and easy to misdiagnose. It can be identified by the following points: (1) Medical history: For patients with a medical history of thyroid nodules or enlarged cervical lymph nodes with BC, the possibility of metastatic cancer should be considered; (2) Imaging findings: Imaging, like US, magnetic resonance spectroscopy, diffusion magnetic resonance, and perfusion CT, provides useful information such as diffuse calcification or invasion of surrounding tissues for assessing the nature of the nodules or lymph nodes[20-22]. However, it is difficult to determine the source of malignancy; (3) Findings of biopsy and immunohistochemical staining: The accuracy of US-guided biopsy for diagnosis of TM is reported to be 90.8% to 91.2%[7]. However, some thyroid metastatic carcinomas can still have similar morphological features to those of primary thyroid cancer by biopsy, including papillary, follicular, and myeloid structures. On these occasions, immunohistochemical staining, such as for TG, calcitonin, GATA3, PAX8, and thyroid transcription factor-1 (TTF-1), is helpful for distinguishing between primary thyroid cancer and TM. TG is the only specific marker for malignant tumors of thyroid origin and can confirm the nonmedullary carcinoma of the thyroid containing a follicular structure[16]. Calcitonin is generally considered to be the most important specific marker for parafollicular cells (C cells) and is considered to be an essential condition for diagnosing medullary carcinoma of C cell origin[19]. TTF-1 regulates the transcription of specific genes in thyroid follicular cells and activates the transcription of Tg and thyroid peroxidase genes in thyroid cancer cells[15]. However, TTF-1 has no specificity because it can also be found in lung cancer, and PAX8 can also be found in kidney cancer and gynecological tumors[8]. GATA3, ER, PR, and HER-2 are markers for BC, and the positive staining for E-cadherin supports the catheter phenotype[8]. BC with ER positivity often metastasizes to the thyroid gland and parathyroid glands, while PR positivity tends to metastasize to the myocardium, gastrointestinal tract, or urinary tract epithelium[9]. However, in this case, the BC exhibited strong positivity for Her-2 and negativity for ER and PR. According to this case, the intraoperative pathology can only suggest a diagnosis of malignant tumors, and the source was confirmed by paraffin sectioning and immunohistochemistry; and (4) positron emission tomography-computed tomography findings: If there is a history of malignant tumors, it should be actively explored. Small lesions can be found, and the general condition of patients can be assessed. This patient refused to perform this test because it was too expensive. Based on these characteristics, we can develop a simple diagnostic flow chart for patients who underwent thyroid tumor biopsy and immunohistochemical staining (Figure 5) that can aid in the diagnosis of patients with suspected TC with a history of BC.
The impact of distant metastasis to the thyroid from BC on prognosis is still unknown. Although TM is a late-stage manifestation of malignant tumors, some patients can achieve relatively satisfactory results in prolonging survival if they can be detected early and treated by surgery-based comprehensive methods[17]. Zhou et al[7] found that thyroidectomy is feasible in patients with only TM after the treatment of primary tumors. Chemotherapy or endocrine therapy may be more effective in patients with extensive systemic metastases, such as lungs and bones. Thyroidectomy and cervical lymph node dissection are often used, followed by radiotherapy and chemotherapy[3]. Metastatic thyroid cancer patients are often accompanied by distant metastasis in other parts of the body, so the specific treatment strategy should be based on the original pathological type and location of the lesions[7]. Because of the limited number of BC patients with TM, the findings in our article may not represent all BC patients with TM. To assess the value of treatment strategy in improving the outcome of patients, studies with a large number of patients and long-term follow-up are warranted.
TM from BC is a rare secondary malignancy. Broad differential diagnosis by biopsy and immunohistochemical analysis needs to be considered.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: International Society of Surgery (by election), Asian Association of Endocrine Surgery (Fellow).
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cihan Y, El-Razek AA, Papadakis M, Isik A S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Li X
| 1. | Inic Z, Martinovic A, Inic M, Pilcevic D, Pupic G. Thyroid gland metastasis from breast cancer: A rare case report and literature overview. Srpski arhiv za celokupno lekarstvo. 2018;146:466-469. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol. 2004;30:583-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Liu YP, Tiu CM, Chou YH, Hsu CY, King KL, Lai YC, Wang HK, Chiou HJ, Chang CY. Thyroid metastasis from breast cancer presenting with diffuse microcalcifications on sonography: a case report. J Clin Ultrasound. 2014;42:430-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf). 2007;66:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Gong Y, Jalali M, Staerkel G. Fine needle aspiration cytology of a thyroid metastasis of metaplastic breast carcinoma: a case report. Acta Cytol. 2005;49:327-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Zhou L, Chen L, Xu D, Shao Q, Guo Z, Ge M. Breast cancer metastasis to thyroid: a retrospective analysis. Afr Health Sci. 2017;17:1035-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Ghias AF, Epps G, Cottrill E, Mardekian SK. Multifocal Metastatic Breast Carcinoma to the Thyroid Gland Histologically Mimicking C Cell Lesions. Case Rep Pathol. 2019;2019:9890716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Yang SI, Park KK, Kim JH. Thyroid metastasis from breast carcinoma accompanied by papillary thyroid carcinoma. Case Rep Oncol. 2014;7:528-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Owens CL, Basaria S, Nicol TL. Metastatic breast carcinoma involving the thyroid gland diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol. 2005;33:110-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Magers MJ, Dueber JC, Lew M, Pang JC, Davenport RD. Metastatic ductal carcinoma of the breast to the thyroid gland diagnosed with fine needle aspiration: A case report with emphasis on morphologic and immunophenotypic features. Diagn Cytopathol. 2016;44:530-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Durmo R, Albano D, Giubbini R. Thyroid metastasis from breast cancer detected by 18F-FDG PET/CT. Endocrine. 2019;64:424-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yang M, Wang W, Zhang C. Thyroid gland metastasis arising from breast cancer: A case report. Oncol Lett. 2013;5:1836-1838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Zeng H, Liu C, Zeng YJ, Wang L, Chen GB, Shen XM. Collision metastasis of breast and thyroid carcinoma to a single cervical lymph node: report of a case. Surg Today. 2012;42:891-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Lacka K, Breborowicz D, Uliasz A, Teresiak M. Thyroid metastases from a breast cancer diagnosed by fine-needle aspiration biopsy. Case report and overview of the literature. Exp Oncol. 2012;34:129-133. [PubMed] [Cited in This Article: ] |
| 16. | Bourcier K, Fermeaux V, Leobon S, Deluche E. Lobular Breast Carcinoma Metastasis to the Thyroid Gland: Case Report and Literature Review. J Breast Cancer. 2018;21:463-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Pensabene M, Stanzione B, Cerillo I, Ciancia G, Cozzolino I, Ruocco R, Condello C, Di Lorenzo G, Giuliano M, Forestieri V, Arpino G, De Placido S, Lauria R. It is no longer the time to disregard thyroid metastases from breast cancer: a case report and review of the literature. BMC Cancer. 2018;18:146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Plonczak AM, DiMarco AN, Dina R, Gujral DM, Palazzo FF. Breast cancer metastases to the thyroid gland - an uncommon sentinel for diffuse metastatic disease: a case report and review of the literature. J Med Case Rep. 2017;11:269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf). 2005;62:236-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol. 2013;82:982-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Razek AA, Tawfik AM, Elsorogy LG, Soliman NY. Perfusion CT of head and neck cancer. Eur J Radiol. 2014;83:537-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Abdel Razek AAK. Routine and Advanced Diffusion Imaging Modules of the Salivary Glands. Neuroimaging Clin N Am. 2018;28:245-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |