Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5304
Peer-review started: June 28, 2020
First decision: July 24, 2020
Revised: July 28, 2020
Accepted: September 29, 2020
Article in press: September 29, 2020
Published online: November 6, 2020
Pancreatic panniculitis is an extremely rare condition associated with different underlying pancreatic disorders and characterized by subcutaneous fat necrosis induced by elevated serum lipase levels. These lesions usually affect the lower extremities and may precede abdominal symptoms of pancreatic disease. Acinar cell carcinoma (ACC) of the pancreas is a rare pancreatic neoplasm, accounting for only 1%-2% of pancreatic tumors in adults.
We present the case of a 72-year-old man with ACC of the pancreatic head and synchronous liver metastases. Both the primary tumor and liver metastases were resected. Serum lipase was elevated before surgery and decreased to normal postoperatively. Rising serum lipase levels at follow-up led to the diagnosis of hepatic recurrence. This disease progression was then accompanied by pancreatic panniculitis, with subcutaneous fat necrosis and acute arthritis. To the best of our knowledge, only 4 cases have been reported in the literature and each showed a similar association of serum lipase levels with pancreatic panniculitis and progression of ACC.
Clinical symptoms and progression of ACC may correlate with serum lipase levels, suggesting potential usefulness as a follow-up biomarker.
Core Tip: This case report highlights the aggressive progress of metastasized pancreatic acinar cell carcinoma in a male patient. The included systematic review lends support to the possibility that increased serum lipase levels with simultaneous pancreatic panniculitis should prompt suspicion for acinar cell carcinoma and lead to further diagnostic steps.
- Citation: Miksch RC, Schiergens TS, Weniger M, Ilmer M, Kazmierczak PM, Guba MO, Angele MK, Werner J, D'Haese JG. Pancreatic panniculitis and elevated serum lipase in metastasized acinar cell carcinoma of the pancreas: A case report and review of literature. World J Clin Cases 2020; 8(21): 5304-5312
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5304.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5304
Pancreatic panniculitis was first described in 1883 and appears in 0.3%-3% of patients with various pancreatic diseases[1-3]. Acinar cell carcinoma (ACC) of the pancreas is equally rare and reported in only 1%-2% of all exocrine pancreatic tumors in adults[4,5]. Hyperlipasemia can occur in patients with ACC of the pancreas[6], since lipase production takes place in the acinar cells. Due to the acinar origin of this tumor, pancreatic panniculitis is observed more frequently in ACC than in any other pancreatic tumor types[7,8]. Lipase hypersecretion can cause fever, polyarthritis, eosinophilia, and progression of fat necrosis to pancreatic panniculitis[9,10], a condition described as “lipase hypersecretion syndrome” by Butturini et al[11].
The combination of symptoms of pancreatitis, panniculitis, and polyarthritis has been summarized as “PPP syndrome” and is associated with a mortality rate of up to 24% in pancreatic cancer[12,13]. Additionally, in patients with PPP syndrome, necrosis may also affect subcutaneous tissue and skeletal structures as well[14]. The hypothesis of the underlying cause of these symptoms is secretion of pancreatic enzymes into the bloodstream[15].
Microscopically, pancreatic panniculitis shows necrosis, neutrophil infiltration, and necrotic adipocytes called “ghost cells”[16]. With regard to treatment options, early resection of the primary AAC tumor is recommended, whenever feasible. This approach may also include resection of synchronous and/or metachronous metastases[17]. Indeed, almost 50% of the patients have evidence of synchronous metastases at the time of diagnosis, and surgical resection is the treatment of choice[18,19].
Evidence for the administration of chemotherapy in ACC is scarce and thus, it is not clear whether patients with ACC might benefit from systemic adjuvant or neoadjuvant regimens. Only small case series have been published on adjuvant chemotherapy[20,21]. Oxaliplatin-based and 5-fluorouracil (5-FU)-based therapeutic protocols seem to show better antitumor activity compared to gemcitabine-based protocols[22]. Yoo et al[19] described oxaliplatin-based regimens in locally advanced and metastatic disease of ACC with significantly improved progression-free survival compared to a gemcitabine-based regimen. Furthermore, Jimbo et al[23] showed 5-FU-based neoadjuvant chemotherapy to be a promising option for locally unresectable ACC.
High recurrence rates have been reported even after curative surgery in ACC[11,18,19]. Overall survival rates for localized disease after curative surgery were reported to be 38 mo[18] and 14 mo[24] in patients with synchronous metastases after surgery. The median survival after resection has been reported to be around almost 2 years[7,20,25].
Collective findings reported in the literature indicate that ACC has a better prognosis than pancreatic ductal adenocarcinoma[17]; however, development of effective treatment strategies with surgery and systemic chemotherapy is a critical aspect for improvement of survival rates. Furthermore, tumor markers may improve early diagnosis and detection of disease recurrence. Kruger et al[22] were the first to describe serum lipase as a marker for prognosis and treatment efficacy in ACC; they showed repeated lipase measurements to monitor the dynamics in 5 patients.
Here, we report the case of a patient with metastatic ACC and pancreatic panniculitis that highlights the dynamics of serum lipase in the individual course of the disease and its value for diagnosis and detection of relapse.
A 72-year-old man was referred to the hospital of the Ludwig-Maximilians-University (Munich, Germany) to address an isolated complaint of abdominal pain.
The patient reported that the abdominal pain had appeared that day and denied chronicity or any other symptoms.
There was no history of past illness.
The physical examination revealed a biologically unremarkable patient in good condition, with only diffuse abdominal pain during palpation.
Routine laboratory tests were unremarkable, except for serum lipase, which was elevated (5580 U/L) and prompted suspicion of ACC.
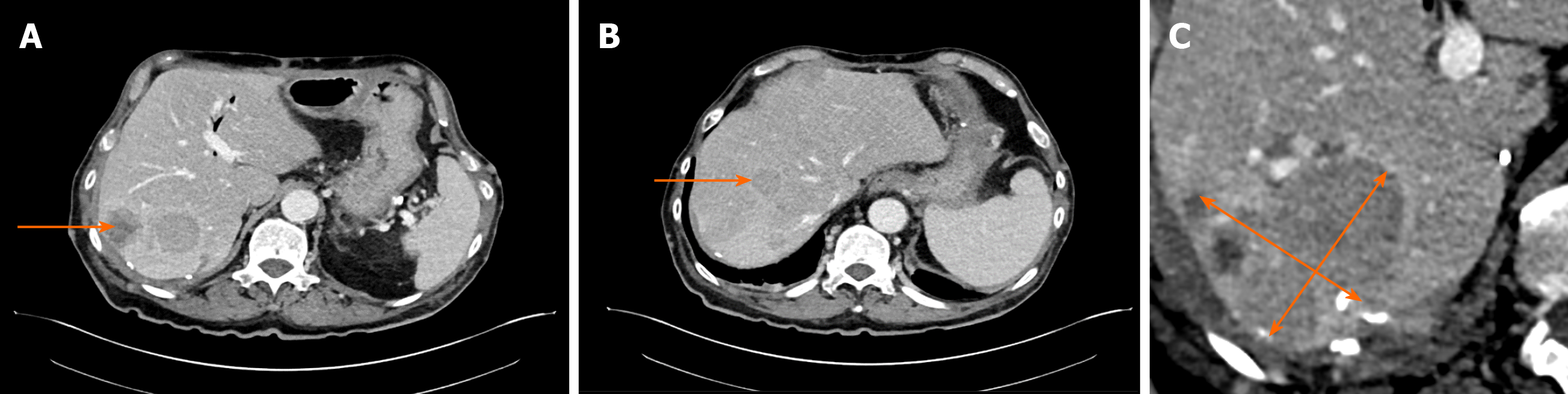
Initial imaging examination with computed tomography (CT) detected tumoral growth of the pancreatic head (Figure 1A) and synchronous liver metastases (Figure 1B).
We presented the case in multidisciplinary expert consultation, which resulted in a diagnosis of pancreatic ACC with synchronous liver metastases. The consensus recommendation was an up-front surgical treatment.
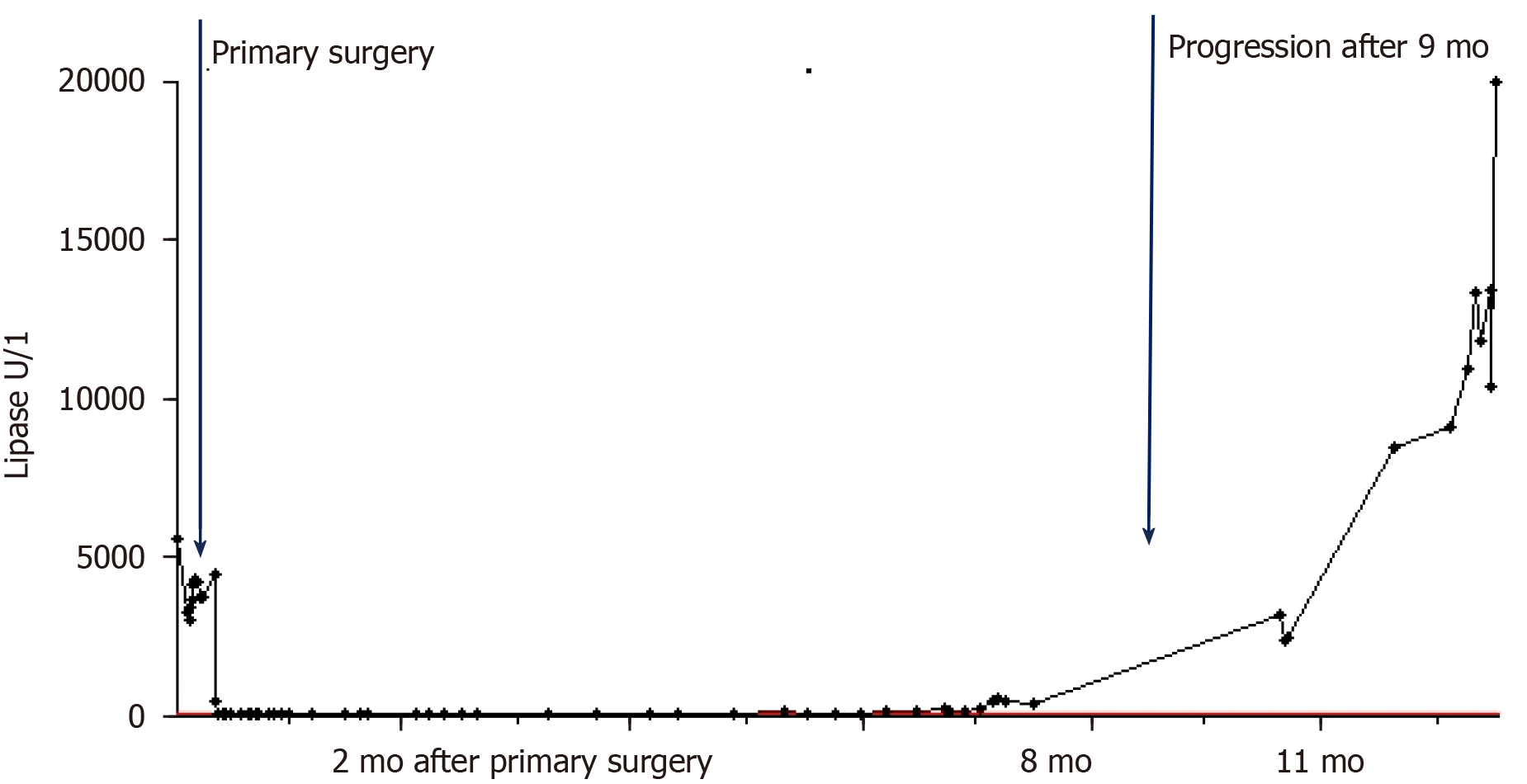
Two-stage surgery was performed, consisting of a pylorus-preserving pancreatoduodenectomy first and right hemihepatectomy 9 d later. After the second surgery, the patient’s serum lipase showed a decrease to 432 U/L. The diagnosis of ACC in the pancreas and the liver was confirmed by pathology. There were no histopathological findings of pancreatitis in the corresponding pancreas tissue.
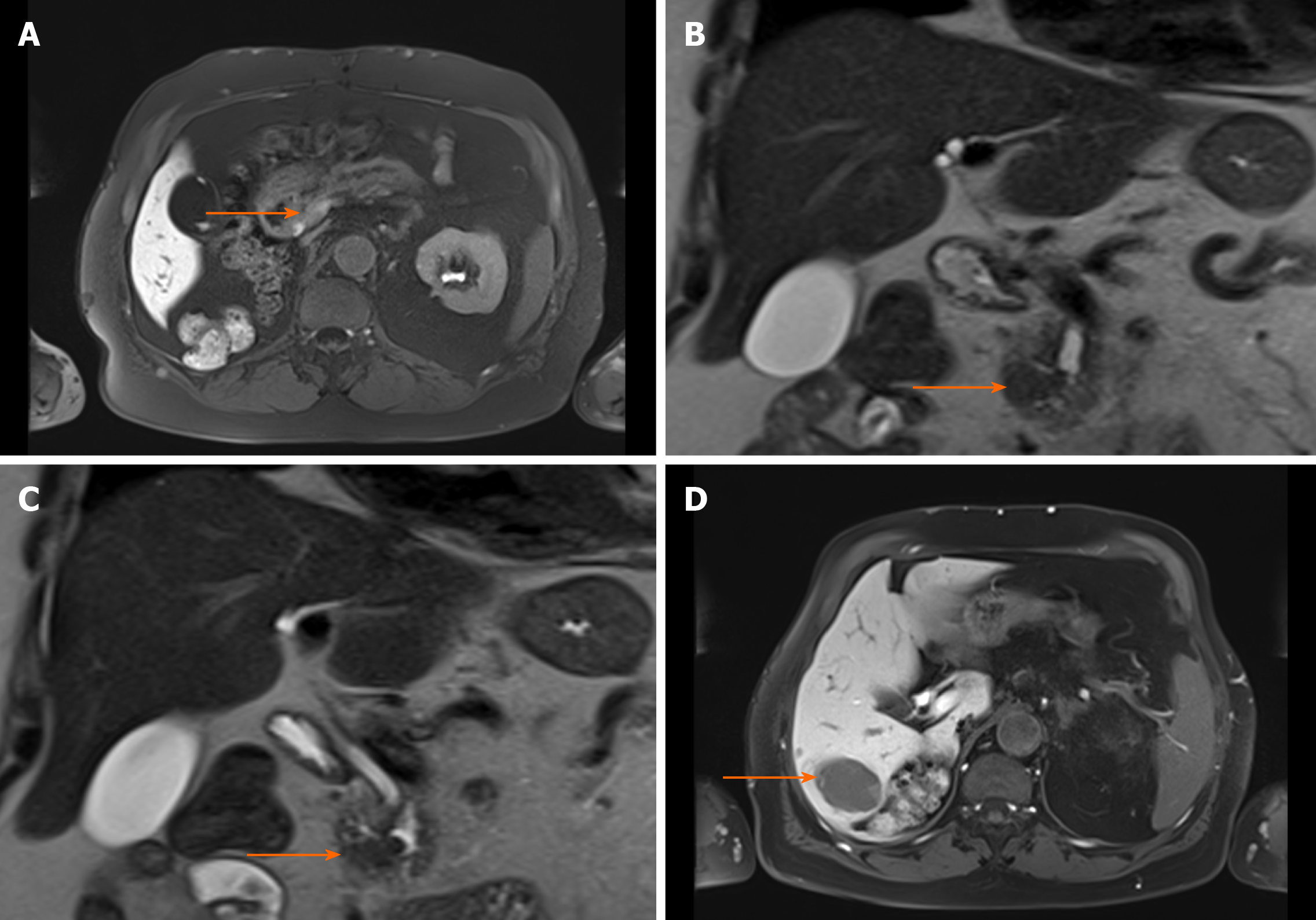
After complete resection of the primary and liver lesions, adjuvant chemotherapy (fluoropyrimidine S-1) was initiated. At 9 mo after the surgery, however, an increase of the serum lipase (to 2384 U/L) was detected. Subsequently, staging diagnostics were performed and hepatic recurrence was found by CT scan (Figure 2). There were no typical signs of pancreatitis as pancreatic inflammation, peripancreatic fluid collection, fluid collections, retroperitoneal air or necrosis. Consequently, a modified FOLFOX6 chemotherapy protocol was recommended.
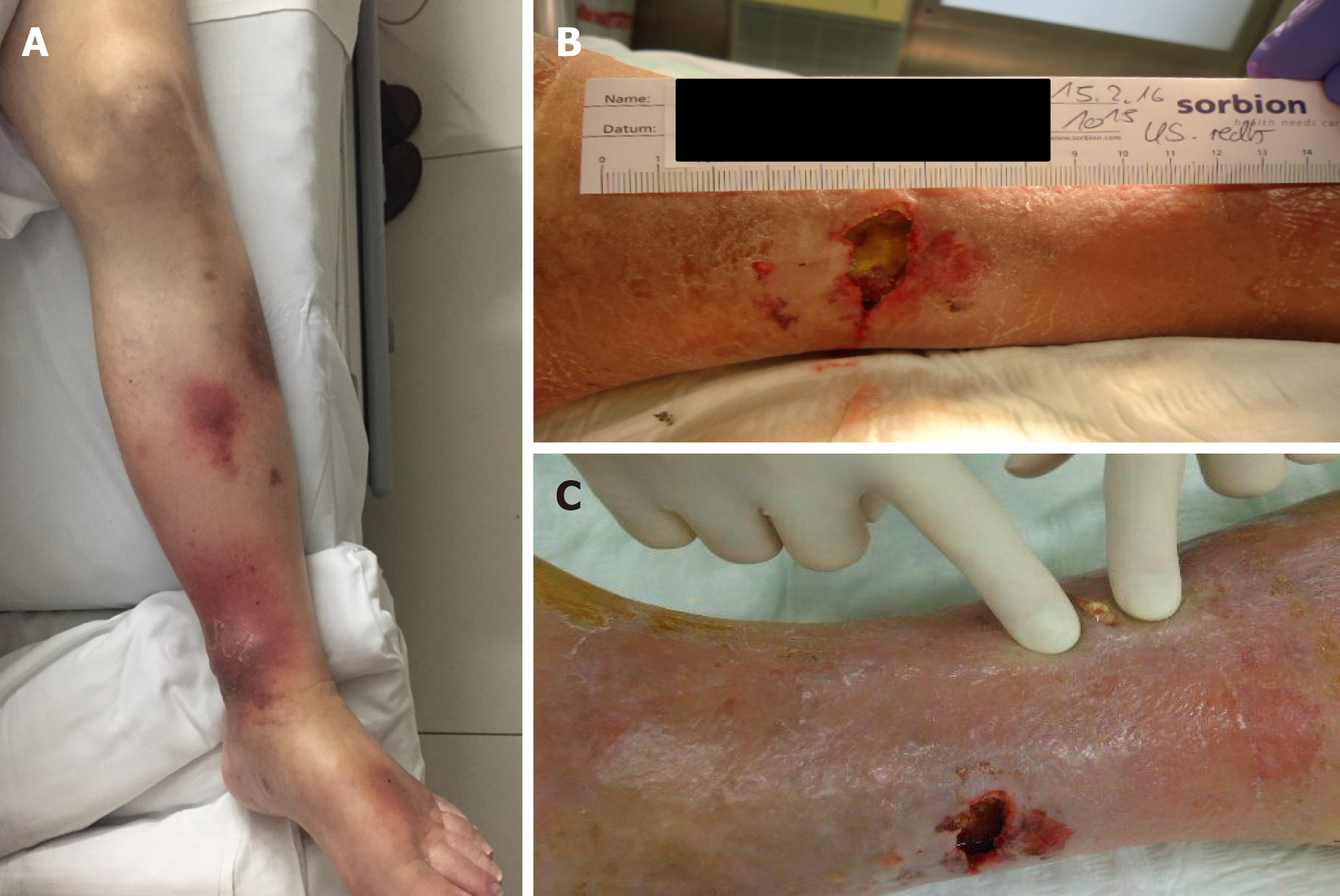
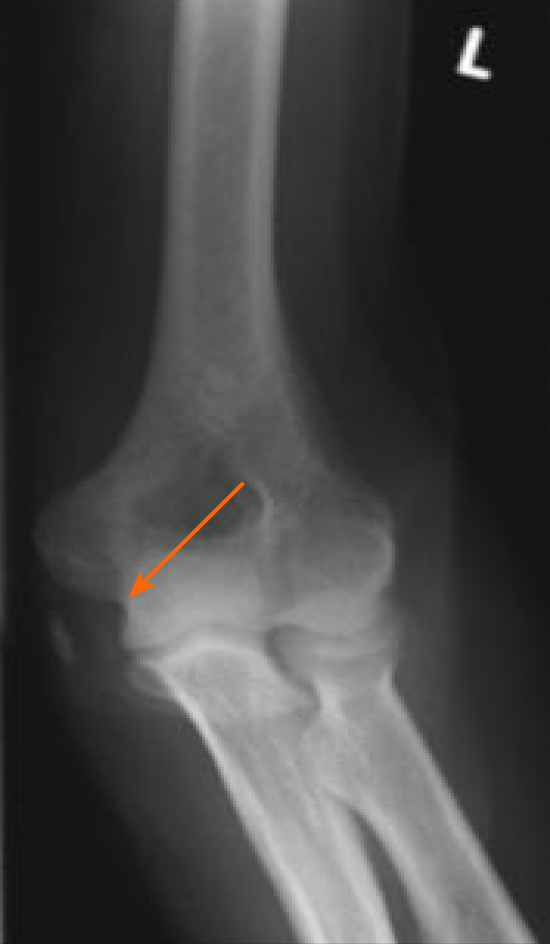
At 10 mo after the initial operation, a further increase of the serum lipase (to 8414 U/L) was detected when tests were prompted by erythema suspicious for panniculitis occurred in both legs (Figure 3). Shortly thereafter, the patient developed necrosis in the lower extremities, which was accompanied by a sharp increase in the serum lipase level (to 10949 U/L). At 1 year after the surgery, arthritis presented in the patient’s left elbow and another increase in serum lipase (of 13340 U/L) was detected (Figure 4). At this time, panniculitis also appeared on the patient’s facial skin and serum lipase reached its highest level (of 19940 U/L) (Figure 5). Meanwhile, there was no relation between the serum lipase levels and the tumor marker levels of carcinoembryonic antigen (CEA) and CA19-9 during follow-up (Figure 5). The patient has passed away 12 mo after first presentation.
A systematic search of PubMed and the Cochrane Library was performed according to the PRISMA guidelines[26]. Medical Subject Headings (commonly known as MeSH) were used as search terms and included “Panniculitis” (MeSH) and “Acinar Cell Carcinoma” (MeSH). A Boolean operator (“AND”) was used to combine the mentioned search terms. Additionally, reference lists were searched for relevant literature. Publications in any language other than English, published before 1995 or without ACC of the pancreas were excluded from further analysis.
The literature search revealed a total of 23 publications, out of which 4 written in non-English language and 3 not reporting data on pancreatic manifestations were excluded. In three[24,27,28] of the sixteen publications[2,3,24,27-39], lipase values corresponding to the clinical course of the patients were described (Table 1). Eight publications showed no correlation between the level of serum lipase and the clinical course[24,27-30,33,37]; these papers described high levels of serum lipase at the stage of diagnosis or surgery but without further assessment of laboratory values.
| Ref. | Age | Location | Metastasis | Pancreatic panniculitis | Serum lipase at time of diagnosis | Monitoring of serum lipase during follow-up | |
| Moro et al[28] | 79 | Head | Liver, metachronous | 12 mo after diagnosis | NA | 1508 U/L postoperatively, normal, 241 U/L (metastasis) | |
| Miksch et al | 72 | Head | Liver, synchronous | 10 mo after diagnosis | 5580 U/L | 432 U/L postoperatively after surgeries, 2384 U/L (hepatic recurrence, 9 mo after surgery), 8414 U/L (pancreatic panniculitis), 10949 U/L, 13340 U/L (arthritis, 12 mo), 19940 U/L (end of follow-up) | |
| Heykarts et al[24] | 61 | Corpus | Liver, synchronous | At diagnosis | NA | Increased, normal, increased (pancreatic panniculitis) | |
| Kuerer et al[27] | 61 | Tail | None | At diagnosis | 17621 U/L | 17621 U/L, 29 U/L postoperatively | |
| 54 | Head | Liver, synchronous | At diagnosis | 710 U/L | 710 U/L, decreased postoperatively | ||
The current knowledge on ACC of the pancreas is based on publications of small case series[19]. If feasible, up-front surgery is recommended[40]. For our case, we performed a two-stage resection of the primary tumor of the pancreas and its hepatic metastases[17,41]. Al-Hader et al[42] endorse resection of the sites of metastasis, to provide better overall survival than that seen in patients without resection.
Evidence for adjuvant chemotherapy is scarce and can only be deduced from small case series[18-23,43]. Yoo et al[19] proposed oxaliplatin-based regimens which may offer a higher efficacy than gemcitabine monotherapy. They showed improved progression-free survival in patients treated with the common FOLFOX- and FOLFIRINOX regimen, respectively[19]. Among the literature, oxaliplatin-based regimens are most commonly used in cases of metastatic ACC of the pancreas[19,22]. Richard et al[44] have described a case of response of pancreatic ACC to anti-endothelial growth factor receptor (EGFR) therapy related to EGFR amplification.
Almost 50% of ACC patients present with synchronous metastases at the stage of diagnosis. Nevertheless, there are recurrence rates of up to 72% after curative surgery of non-metastasized ACC[23]. Thus, up-front surgery remains the recommended treatment option for patients with either metastasized or non-metastasized ACC. Subsequent therapies must also be considered.
Pancreatic panniculitis as an extra-pancreatic manifestation and most frequently described in cases of ACC[7,8]. It usually affects the lower extremities[31,45], where most subcutaneous tissue is located. The occurrence of pancreatic panniculitis varies from patient to patient. Herein, we have described a patient with pancreatic panniculitis at almost 1 year after primary surgery. This is similar to the findings of Ferri et al[46] and Zhang et al[47], who reported occurrence of pancreatic panniculitis with serum lipase values of 3885 U/L and 27575 U/L, respectively; neither of those case reports mentioned abdominal symptoms at that time of elevated serum lipase. The combination of subcutaneous panniculitis and elevated serum lipase may be an important clue to suspect recurrent ACC at follow-up[12].
Our patient presented a combination of clinical symptoms with panniculitis and polyarthritis without signs of pancreatitis in several CT scans. Whereas Ferri et al[46] showed that half of the reviewed 64 cases with PPP syndrome occurred in patients with pancreatic ductal adenocarcinoma, whereas 34 cases were reported for patients with pancreatitis.
Impact of serum lipase on the pathogenesis of the PPP syndrome has been verified in mouse models[10]. The systemic pancreatic enzyme secretion causes systemic fat necrosis, especially in subcutaneous fat tissue[35]. In all reported cases, an exceptionally high level of lipase was found. Other pancreatic enzymes, such as amylase, phosphorylase and trypsin, may play a role in the development of a hyperlipasemia syndrome, itself consisting of fever, arthralgia, rash, nodular panniculitis, and hypereosinophilia[9-11]. The clinical symptoms of fever, arthritis, and pancreatic panniculitis were detected in our patient during disease progression of the ACC.
For the case presented herein, the serum lipase correlated closely with the clinical course of ACC and pancreatic panniculitis. In this respect, disease progression was detected postoperatively via increasing levels of the serum lipase and subsequent radiological detection by CT scan. As demonstrated by our and other cases, serum lipase might indeed be a good tumor marker of disease progression in ACC[22,24,28].
In our case of synchronous metastasized ACC, serum lipase was a reliable marker for tumor diagnosis, recurrence, and progression. Therefore, measurement of serum lipase may help to predict prognosis and monitor the individual course of the disease in ACC patients.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Deutsche Gesellschaft für Chirurgie; European Digestive Surgery; Deutscher Pankreasclub; European Pancreatic Club; and International Association of Pancreatology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsuo Y S-Editor: Huang P L-Editor: A P-Editor: Li JH
| 1. | Chiari H. Über die sogenannte Fettnekrose. Prag Med Wochenschr. 1883;8:255-256. [Cited in This Article: ] |
| 2. | Callata-Carhuapoma HR, Pato Cour E, Garcia-Paredes B, Fernandez RM, Mendoza Fernandez ML, Fernandez AM, De La Rosa CA, Sotelo Lezama MJ, Cabezas-Camarero S, Sastre Varela J. Pancreatic acinar cell carcinoma with bilateral ovarian metastases, panniculitis and polyarthritis treated with FOLFIRINOX chemotherapy regimen. A case report and review of the literature. Pancreatology. 2015;15:440-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 3. | Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425. [PubMed] [Cited in This Article: ] |
| 4. | Toll AD, Mitchell D, Yeo CJ, Hruban RH, Witkiewicz AK. Acinar cell carcinoma with prominent intraductal growth pattern: case report and review of the literature. Int J Surg Pathol. 2011;19:795-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20 Suppl 1:S94-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 334] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014;31:491-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Jang SH, Choi SY, Min JH, Kim TW, Lee JA, Byun SJ, Lee JW. [A case of acinar cell carcinoma of pancreas, manifested by subcutaneous nodule as initial clinical symptom]. Korean J Gastroenterol. 2010;55:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, Yu RT, Olefsky JM, Henry RR, Downes M, Evans RM. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Butturini G, Pisano M, Scarpa A, D'Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011;396:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Narváez J, Bianchi MM, Santo P, de la Fuente D, Ríos-Rodriguez V, Bolao F, Narváez JA, Nolla JM. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Boswell SH, Baylin GJ. Metastatic fat necrosis and lytic bone lesions in a patient with painless acute pancreatitis. Radiology. 1973;106:85-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kang DJ, Lee SJ, Choo HJ, Her M, Yoon HK. Pancreatitis, panniculitis, and polyarthritis (PPP) syndrome: MRI features of intraosseous fat necrosis involving the feet and knees. Skeletal Radiol. 2017;46:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Dieker W, Derer J, Henzler T, Schneider A, Rückert F, Wilhelm TJ, Krüger B. Pancreatitis, panniculitis and polyarthritis (PPP-) syndrome caused by post-pancreatitis pseudocyst with mesenteric fistula. Diagnosis and successful surgical treatment. Case report and review of literature. Int J Surg Case Rep. 2017;31:170-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Laureano A, Mestre T, Ricardo L, Rodrigues AM, Cardoso J. Pancreatic panniculitis - a cutaneous manifestation of acute pancreatitis. J Dermatol Case Rep. 2014;8:35-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hartwig W, Denneberg M, Bergmann F, Hackert T, Hinz U, Strobel O, Büchler MW, Werner J. Acinar cell carcinoma of the pancreas: is resection justified even in limited metastatic disease? Am J Surg. 2011;202:23-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673-4678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Yoo C, Kim BJ, Kim KP, Lee JL, Kim TW, Ryoo BY, Chang HM. Efficacy of Chemotherapy in Patients with Unresectable or Metastatic Pancreatic Acinar Cell Carcinoma: Potentially Improved Efficacy with Oxaliplatin-Containing Regimen. Cancer Res Treat. 2017;49:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Glazer ES, Neill KG, Frakes JM, Coppola D, Hodul PJ, Hoffe SE, Pimiento JM, Springett GM, Malafa MP. Systematic Review and Case Series Report of Acinar Cell Carcinoma of the Pancreas. Cancer Control. 2016;23:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Wang S, Zhou X, Zhou H, Cui Y, Li Q, Zhang L. Acinar cell carcinoma: a report of 19 cases with a brief review of the literature. World J Surg Oncol. 2016;14:172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Kleespies A, Angele MK, Hartwig W, Bruns CJ, Kirchner T, Werner J, Heinemann V, Boeck S. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142:2585-2591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Jimbo M, Batista PM, Baliff JP, Yeo CJ. Neoadjuvant Chemotherapy and Appleby Procedure for Pancreatic Acinar Cell Carcinoma: A Case Report. Case Rep Pancreat Cancer. 2016;2:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Heykarts B, Anseeuw M, Degreef H. Panniculitis caused by acinous pancreatic carcinoma. Dermatology. 1999;198:182-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078-2086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47017] [Cited by in F6Publishing: 43253] [Article Influence: 2883.5] [Reference Citation Analysis (0)] |
| 27. | Kuerer H, Shim H, Pertsemlidis D, Unger P. Functioning pancreatic acinar cell carcinoma: immunohistochemical and ultrastructural analyses. Am J Clin Oncol. 1997;20:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Moro M, Moletta L, Blandamura S, Sperti C. Acinar cell carcinoma of the pancreas associated with subcutaneous panniculitis. JOP. 2011;12:292-296. [PubMed] [Cited in This Article: ] |
| 29. | Durden FM, Variyam E, Chren MM. Fat necrosis with features of erythema nodosum in a patient with metastatic pancreatic carcinoma. Int J Dermatol. 1996;35:39-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Feuer J, Spiera H, Phelps RG, Shim H. Panniculitis of pancreatic disease masquerading as systemic lupus erythematosus panniculitis. J Rheumatol. 1995;22:2170-2172. [PubMed] [Cited in This Article: ] |
| 31. | Beltraminelli HS, Buechner SA, Häusermann P. Pancreatic panniculitis in a patient with an acinar cell cystadenocarcinoma of the pancreas. Dermatology. 2004;208:265-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Poelman SM, Nguyen K. Pancreatic panniculitis associated with acinar cell pancreatic carcinoma. J Cutan Med Surg. 2008;12:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Iwatate M, Matsubayashi H, Sasaki K, Kishida N, Yoshikawa S, Ono H, Maitra A. Functional pancreatic acinar cell carcinoma extending into the main pancreatic duct and splenic vein. J Gastrointest Cancer. 2012;43:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Lakhani A, Maas L. Necrotizing panniculitis: a skin condition associated with acinar cell carcinoma of the pancreas. South Med J. 2008;101:554-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Arbeláez-Cortés A, Vanegas-García AL, Restrepo-Escobar M, Correa-Londoño LA, González-Naranjo LA. Polyarthritis and pancreatic panniculitis associated with pancreatic carcinoma: review of the literature. J Clin Rheumatol. 2014;20:433-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Banfill KE, Oliphant TJ, Prasad KR. Resolution of pancreatic panniculitis following metastasectomy. Clin Exp Dermatol. 2012;37:440-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol. 2010;9:1145-1150. [PubMed] [Cited in This Article: ] |
| 38. | Gorovoy IR, McSorley J, Gorovoy JB. Pancreatic panniculitis secondary to acinar cell carcinoma of the pancreas. Cutis. 2013;91:186-190. [PubMed] [Cited in This Article: ] |
| 39. | Stauffer JA, Bray JM, Nakhleh RE, Bowers SP. Image of the month. Acinar cell carcinoma. Arch Surg. 2011;146:1099-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, O'Reilly EM. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Distler M, Rückert F, Dittert DD, Stroszczynski C, Dobrowolski F, Kersting S, Grützmann R. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J Surg Oncol. 2009;7:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol. 2017;23:7945-7951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Richard C, Niogret J, Boidot R, Ghiringhelli F. EGFR amplification induces sensitivity to antiEGFR therapy in pancreatic acinar cell carcinoma. World J Gastrointest Oncol. 2018;10:103-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Menzies S, McMenamin M, Barnes L, O'Toole D. Pancreatic panniculitis preceding acute pancreatitis and subsequent detection of an intraductal papillary mucinous neoplasm: A case report. JAAD Case Rep. 2016;2:244-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Ferri V, Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Malave L, Plaza C, Rodriguez S, Garcia L, Perez V, Quijano Y, Vicente E. Pancreatic disease, panniculitis, polyarthrtitis syndrome successfully treated with total pancreatectomy: Case report and literature review. Int J Surg Case Rep. 2016;28:223-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Zhang G, Cao Z, Yang G, Wu W, Zhang T, Zhao Y. Pancreatic panniculitis associated with pancreatic carcinoma: A case report. Medicine (Baltimore). 2016;95:e4374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Sumiyoshi T, Shima Y, Okabayashi T, Kozuki A, Nakamura T. Comparison of pancreatic acinar cell carcinoma and adenocarcinoma using multidetector-row computed tomography. World J Gastroenterol. 2013;19:5713-5719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |