Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5284
Peer-review started: May 6, 2020
First decision: August 23, 2020
Revised: September 5, 2020
Accepted: September 16, 2020
Article in press: September 16, 2020
Published online: November 6, 2020
Anterior bone loss (ABL) is a relatively easily neglected condition after cervical disc replacement (CDR). Whether this phenomenon is a radiological anomaly or a complication remains controversial. Several studies have reported the clinical characteristics of ABL and speculated on the pathogenic mechanism based on a certain type of artificial disc, while the overall understanding of ABL is lacking.
To describe the prevalence, impacts, and risk factors of ABL after CDR.
We searched the PubMed, Cochrane Library, and Excerpta Medica databases using the terms “bone loss” or “bone remodeling” or “bone absorption” or “osteolysis” or “implant loosening” or “implant migration” or “hypersensitivity” or “hyperreactivity”, “cervical disc replacement” or “cervical disc arthroplasty” or “total disc replacement”. Eligible manuscripts on the prevalence and impacts of ABL were reviewed by the authors. Data extraction was performed using an established extraction form. The results of the included studies were described narratively.
Six studies met the inclusion and exclusion criteria. One was a prospective study and the others were retrospective studies. A total of 440 patients with 536 segments were included. The artificial cervical discs included Bryan, Baguera-C, Discocerv, and Mobi-C. The prevalence of ABL ranged from 3.13% to 91.89%, with a combined overall prevalence of 41.84%. ABL occurred within 6 mo and stopped 12 mo after surgery. Several cases were noted to have a self-healing process. Severe ABL resulted in segmental kyphosis, implant subsidence, and persistent neck pain. ABL may be related to heterotopic ossification. Multilevel surgery may be one of the risk factors for ABL.
ABL is a common condition after CDR. The underlying mechanisms of ABL may include stress concentration and injury to nutrient vessels. ABL should be considered a complication after CDR as it was associated with neck pain, implant subsidence, and heterotopic ossification.
Core Tip: Anterior bone loss (ABL) is a common condition after cervical disc replacement. Several studies have reported the clinical characteristics of ABL; however, it remains unclear whether this phenomenon is a radiological anomaly or a complication. In this review, we found that the prevalence of ABL after cervical disc replacement was related to the type of implant. ABL should be considered a complication after cervical disc replacement as it was associated with neck pain, implant subsidence, and heterotopic ossification. Fortunately, ABL did not progress after 12 mo postoperatively, and some cases showed a self-healing phenomenon. The underlying mechanisms of ABL may include stress concentration and injury to nutrient vessels.
- Citation: Wang XF, Meng Y, Liu H, Hong Y, Wang BY. Anterior bone loss after cervical disc replacement: A systematic review. World J Clin Cases 2020; 8(21): 5284-5295
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5284.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5284
Anterior cervical discectomy and fusion (ACDF) is recognized as the standard procedure for cervical degenerative disc disease. However, concerns such as non-union, pseudarthrosis, and acceleration of adjacent segment degeneration have been raised[1-4]. Compared with ACDF, cervical disc replacement (CDR), a motion preservation technique, can reduce intervertebral disc pressure in adjacent levels and better simulate the physiological condition of the cervical spine[5,6]. Studies have shown that CDR and ACDF have equivalent efficiency in clinical outcomes such as symptom relief[7,8]. However, CDR is better in some respects, such as faster recovery, higher patient satisfaction, and is more cost-effective[9,10].
Although CDR is widely performed and patients have benefited due to its satisfying results, it has some implant-related complications, such as heterotopic ossification (HO), implant migration, and implant subsidence[11-16]. With regard to these complications, in-depth studies have been performed to determine the potential risk factors and clinical impacts[17-20]. In contrast to excessive bone formation (which is HO), anterior bone loss (ABL) is another implant-related condition after CDR and has gained more attention in recent years[21]. ABL is the process of bone rebuilding in the ventral part of vertebral bodies, and can usually be recognized from lateral radiographs. Peri-prosthetic bone loss has been extensively described in large joint arthroplasty. However, ABL is easily ignored in cervical disc arthroplasty.
The prevalence of ABL after CDR varies from 3.13% to 91.89% and differs greatly among different types of artificial cervical discs[22-27]. Several studies have reported the clinical characteristics of ABL and speculated the pathogenic mechanism based on a certain type of artificial disc, while the overall understanding of ABL after CDR is lacking. In addition, it remains controversial whether ABL is a radiological anomaly or a complication. Therefore, we performed this systematic review of currently available clinical data to comprehensively describe the prevalence, impacts, and risk factors of ABL after CDR.
A comprehensive literature search was carried out in PubMed, Cochrane Library, and the Excerpta Medica databases. Studies before May 2019 were included. The following keywords were used in the search: “bone loss” or “bone remodeling” or “bone absorption” or “osteolysis” or “implant loosening” or “implant migration” or “hypersensitivity” or “hyperreactivity”, “cervical disc replacement” or “cervical disc arthroplasty” or “total disc replacement”. Articles published in English that involved peri-prosthetic bone absorption after CDR were specifically identified. The references of all identified papers were manually reviewed to identify further potentially relevant studies.
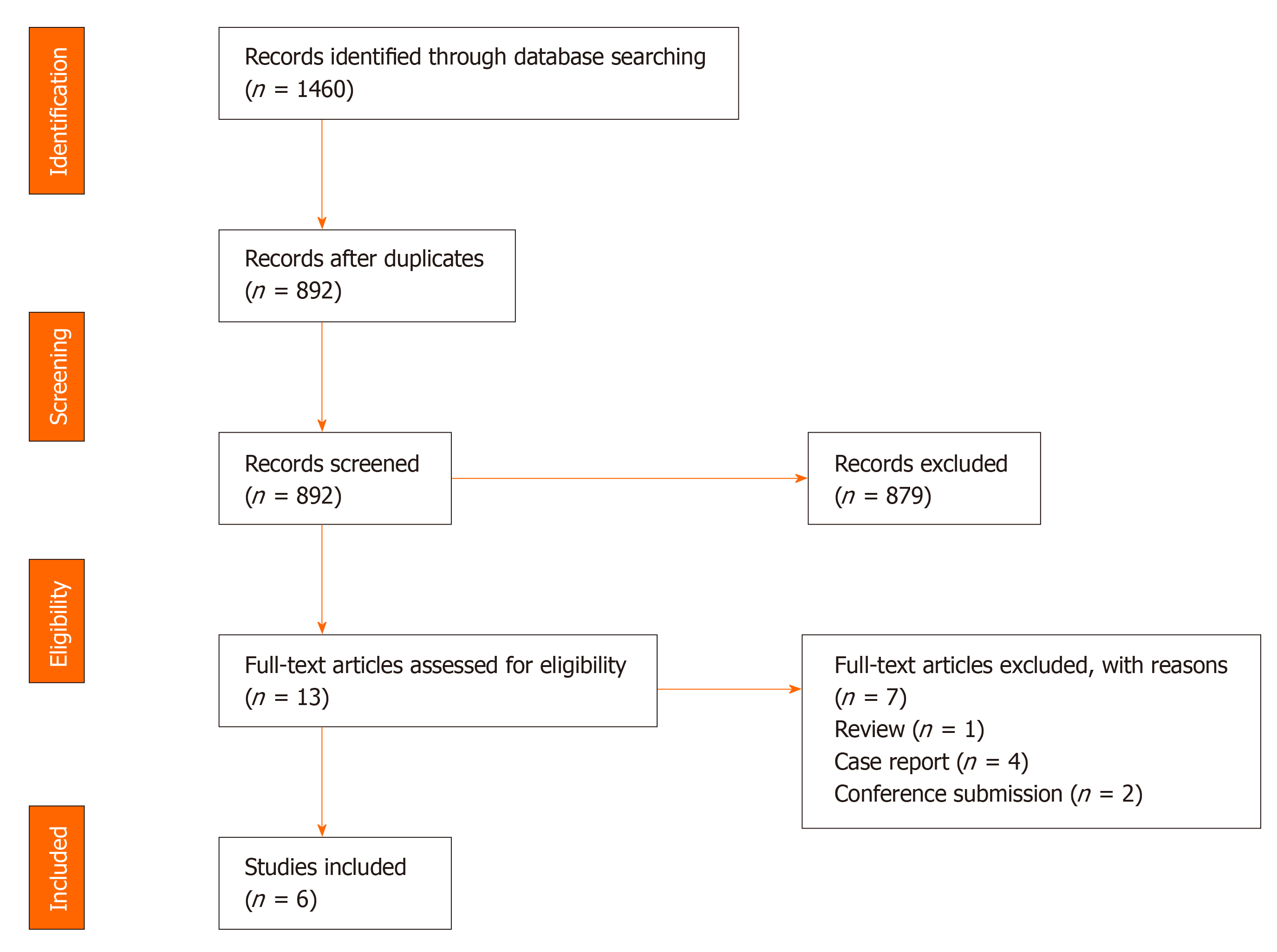
The search produced 1460 published articles. We then systematically assessed the selected articles. The inclusion criteria were as follows: (1) peri-prosthetic bone loss occurred in the surgical segment after CDR; and (2) bone remodeling occurred in the anterior/ventral part of vertebral bodies. The exclusion criteria were as follows: (1) Peri-prosthetic bone loss occurred in another part of the vertebral bodies, (2) Case reports, (3) Reviews, (4) Commentaries, and (5) Cadaveric or experimental studies. The results of the literature search are shown in Figure 1.
The screening of titles and abstracts was performed by two reviewers (Wang XF and Meng Y) independently. Articles that met the eligibility criteria on the first screening were further assessed by reading the full text. After screening, six clinical studies were included in the systematic review. The two reviewers (Wang XF and Meng Y) then performed data extraction and rated the level of evidence of each article independently using the published guideline[28]. The results of this study were described narratively.
One randomized controlled study and five retrospective studies were included in this review[22-27]. A total of 440 patients with 536 segments were included. The mean age of these patients ranged from 45.3 to 50.1 years, and the mean follow-up time ranged from 32.3 to 74.4 mo. The artificial cervical discs included Bryan, Baguera-C, Discocerv, and Mobi-C. The surgical procedure included single-level CDR, double-level CDR, triple-level CDR, and hybrid surgery. Study information and level of evidence are listed in Table 1. Detailed information on ABL including the prevalence, course, effects, and outcomes are summarized in Tables 2-4 and Figure 2.
| Ref. | Yr | Study type | Level of evidence | Number and type of artificial disc | Sex | Age (yr) | Follow-up |
| Ren et al[22] | 2011 | Prospective and nonrandomized study | 3 | Total (Bryan): 45 patients and 51 implants | 19 Female; 26 Male | 46 (31-50) | Mean 35 (24-70) mo |
| Hacker et al[23] | 2013 | Randomized controlled study | 2 | Total 51 patientsBryan: 32 patientsPrestige-LP: 19 patients | 27 Female; 24 Male | - | 1, 6, 12, 24 wk and 12, 24, 48, 60 mo |
| Kim et al[24] | 2015 | Prospective registry with retrospective analysis | 3 | Total (Bryan): 37 patients | 13 Female; 24 Male | 45.4 (27-55) | 1, 3, 6, 12, 24, 36 mo, mean 60.1 (42-113) mo |
| Heo et al[25] | 2017 | Retrospective observational study | 3 | Total (Baguera-C): 48 patients | 30 Female; 18 Male | 50.1 ± 7.4 | 1, 6, 12, 24 mo, mean 32.3 ± 3.3 mo |
| Kieser et al[26] | 2018 | Retrospective observational study | 3 | Total 145 patients and 193 implants Bryan: 32 patients, 56 implants, Discocerv: 38 patients, 44 implants; Baguera-C: 44 patients, 56 caudal implants; Mobi-C: 31 patients, 37 implants | 78 Female; 67 Male | 45 (25-65) | 3, 6, 12, 24, 36, 48, 60 mo, mean 6.2 (5-10) yr |
| Kieser et al[27] | 2019 | Retrospective observational study | 3 | Total 114 patients and 156 implants; Bryan: 32 patients, 56 implants; Discocerv: 38 patients, 44 implants; Baguera-C: 44 patients, 56 caudal implants | 65 Female; 49 Male | 45.3 (28-65) | 6 wk, 3, 6, 9, 12, 24 mo, 5 yr, maximum 8 yr |
| Implant | Prevalence | Course | Effect | Outcome |
| Total | 41.84% (159/380) | Occur within 3 mo postop; Does not progress after 12 mo postop; Self-healing in several cases | Higher VAS score within 12 mo postop in severe cases; Exposure or subsidence of implants in severe cases | No significant difference compared with patients without bone loss at the last follow-up |
| Bryan | 22.16% (39/176) | |||
| Discocerv | 47.73% (21/44) | |||
| Baguera-C | 62.50% (65/104) | |||
| Mobi-C | 91.89% (34/37) |
| Ref. | Prevalence | Notification time | Course of ABL | Grading system |
| Ren et al[22] | Bryan: 5.88% more cases were noted from the figures | Within 6 mo postop | Stopped after 6 mo postop | Not reported; ABL distance: < 2 mm in 2 patients, > 2 mm in 1 patient |
| Hacker et al[23] | Total: 1.96%, Bryan: 3.13%; Prestige-LP: 0 | 6 wk postop | Not reported | Not reported |
| Kim et al[24] | Bryan: 8.11% | 3-6 mo postop | Progressed within 6 mo postop; Self-limited | Not reported; Mean ABL distance: 2.57 mm (range 2.0-3.0 mm) |
| Heo et al[25] | Baguera-C: 60.42% | 6 mo postop | Progressed within 12 mo postop; Self-limited | Grade 1, Minor, disappearance of the anterior osteophyte or small minor bone loss: 31.25%; Grade 2, Minor, bone loss of the anterior portion of the vertebral bodies at the operated segment without exposure of the implant: 12.5%; Grade 3, Major, significant bone loss with exposure of the anterior portion of the implant: 16.67% |
| Kieser et al[26] | Total: 63.73%; Cranial: 62.11%; Caudal: 48.7%; Bryan: 57.14%; Discocerv: 47.73%; Baguera-C: 64.29%; Mobi-C: 91.89% | Not reported | Self-limited within 12 mo postop | Mild, ABL ≤ 5%: 48.7%; Moderate, 5% < ABL ≤ 10%: 11.92%; Severe, ABL > 10%: 3.11% |
| Kieser et al[27] | Total: 57.05%; Cranial: 54.9%; Caudal: 42.31%; Bryan: 57.14%; Discocerv: 47.73%; Baguera-C: 64.29% | Within 3 mo postop | Benign course; progressed within 12 mo postop then stopped; self-healing in several cases | Grade 1, Mild, ABL ≤ 5%: 45.51%; Grade 2, Moderate, 5% < ABL ≤ 10%: 8.33%; Grade 3, Severe, ABL > 10% without endplate collapse: 0.64%; Grade 4, Severe, ABL > 10% with endplate collapse: 2.56% |
| Ref. | Radiological effects | Clinical effects | Treatment | Potential risk factors |
| Ren et al[22] | Grade 4 HO in 1 patient with > 2 mm ABL distance, grade 2 HO in 1 patient; only 3 patients had HO in this study | No effect | Monitored | Micromovement of implant |
| Hacker et al[23] | Kyphosis of shell angle and FSU angle; Implant subsidence | Recurrent neck and arm pain persisting 52 mo postop | Revision fusion surgery at index and lower adjacent level | Low virulence bacterial infection of endplates at arthroplasty level |
| Kim et al[24] | Ossification of ALL at the inferior adjacent level in 1 patient | No effect | Monitored | Stress shielding; Friction and wear debris between the anterior flange and vertebra (less likely) |
| Heo et al[25] | No effect | Grade 3 ABL had significantly higher mean VAS score for neck pain at 6 mo (5.3 vs 1.8) and 12 mo (4.7 vs 1.8) postop | Monitored | Stress shielding;good motion function of implants |
| Kieser et al[26] | A significant relationship with HO | A lower NDI score 5 yr postop (P < 0.1) | Not reported | More operative levels; less of traction of ALL; surgical exposure |
| Kieser et al[27] | Exposure or subsidence of implant in severe ABL | No effect | Monitored | Grade 1-2: direct anterior vertebral injury including heat necrosis or resected ALL; Grade 3-4: avascular necrosis caused by injury to nutrient vessels |
ABL was identified on lateral X-rays in all six studies, and had the following two signs: first, the ventral-inferior part of the cranial vertebra or the ventral-superior part of the caudal vertebra at the arthroplasty level disappeared; second, the cortical bone margin of the ABL area still existed and shifted dorsally.
The prevalence of ABL ranged from 3.13% to 91.89%, with a combined overall prevalence of 41.84%, and it differed depending on the implant type. The Mobi-C showed the highest prevalence of ABL (91.89%), followed by the Baguera-C (60.42%-64.29%, 62.50% after combination), Discocerv (47.73%) and Bryan (3.13%-57.14%, 22.16% after combination). In the study by Kieser et al[26], the cranial vertebra showed a higher ABL rate.
ABL occurred within 3-6 mo postoperatively and did not progress after 12 mo postoperatively in most cases. Kieser et al[26,27] reported a “self-healing phenomenon” of ABL in several cases, but they did not present other radiological evidence to prove that the self-healing was not caused by HO. In the study by Hacker, one patient with ABL received revision fusion surgery to relieve symptoms[23].
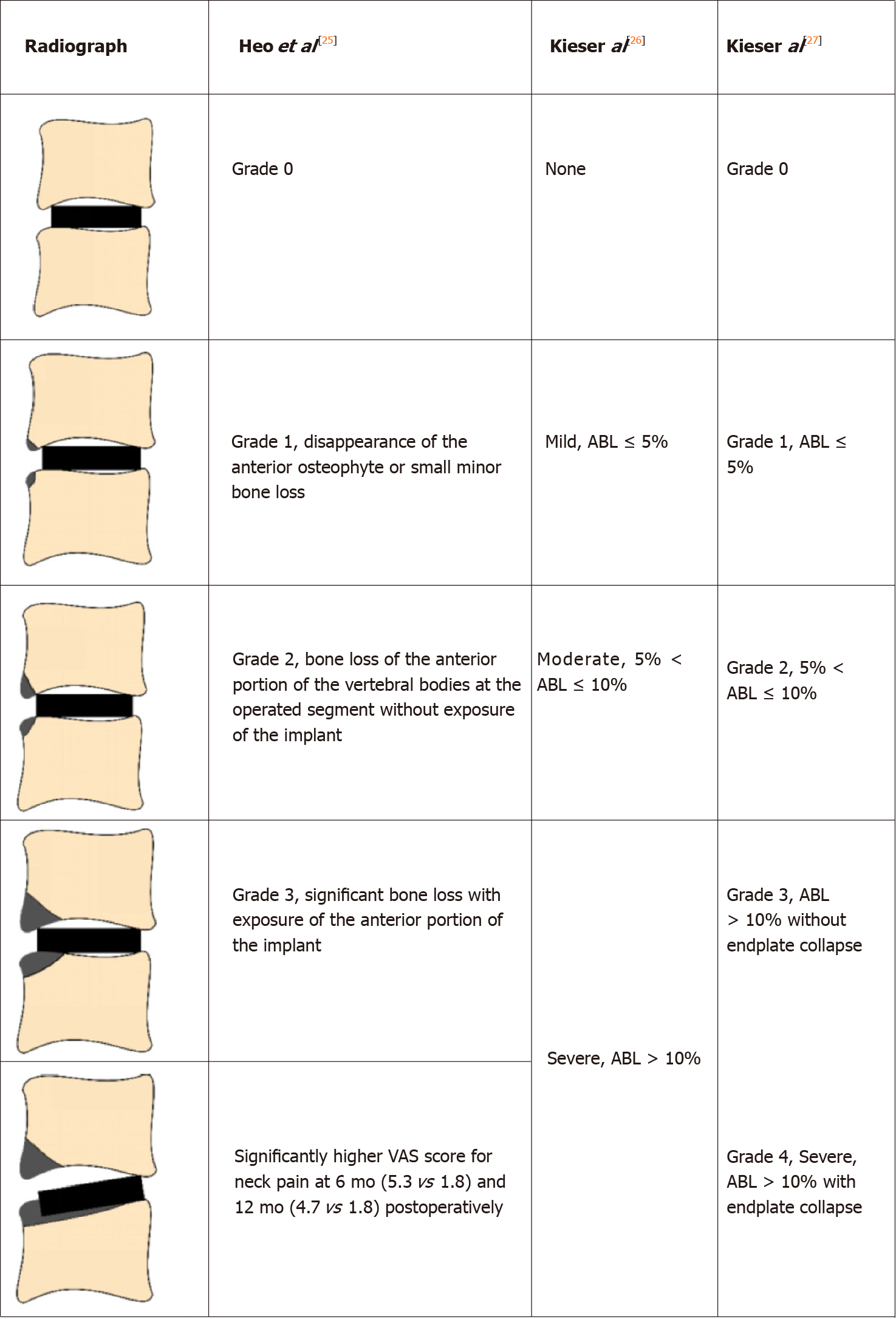
Three studies reported the grading system of ABL based on the lateral radiographs (Table 2). Heo et al[25] divided the ABL into three grades: Grade 1 refers to a small part of the vertebra with ABL; Grade 2 indicates a larger portion of the vertebra with ABL compared with Grade 1, but the implant was not exposed; Grade 3 refers to significant bone loss with exposure of the implant. This non-quantitative grading method is ambiguous when classifying Grade 1 and 2 ABL. The authors then assigned Grade 1 and 2 ABL into the minor change Group (without exposure of implants), and assigned Grade 3 into the major change group (with implant exposure). The prevalence of ABL was 43.75% in the minor change group and 16.67% in the major change group. This grading system is not suitable for artificial discs with an anterior flange such as Bryan, because a small portion of ABL would cause implant exposure. Besides, an obvious ABL may be assigned to the minor change group due to the posteriorly positioned implant.
Kieser et al[26] introduced a quantitative grading method based on the lateral radiographs. They calculated the ratio of the bone loss distance and the initial length of the endplate and then divided the ABL into three groups: mild (ratio ≤ 5%), moderate (5% < ratio ≤ 10%), and severe (ratio > 10%). The prevalence of ABL was 48.7% in the mild group, 11.92% in the moderate group, and 3.11% in the severe group, respectively. Severe ABL caused implant exposure or subsidence.
In 2019, Kieser et al[27] slightly revised their grading system. They divided severe ABL into two subgroups: severe with endplate collapse (four patients), and severe without endplate collapse (one patient, who showed a self-healing process). However, in their grading system, there was no significant difference among the groups in terms of radiological or clinical outcomes. Therefore, this grading system is not instructive in clinical work.
All patients with ABL achieved similar clinical outcomes at the last follow-up, compared with those who did not develop ABL. In the study by Ren et al[22], three patients had ABL, and they did not complain of any subjective discomfort. Two patients had ABL < 2 mm and Grade 2 HO was observed in one of them; one patient had ABL > 2 mm, and grade 4 HO was noted in this patient. It is noteworthy that the authors only observed HO in three patients in this study.
Hacker et al[23] reported one patient with ABL who suffered from recurrent neck and arm pain after surgery for 52 mo. The shell angle was kyphosis at the last follow-up, as well as the functional spine unit angle. This patient received revision surgery, in which the Bryan disc was removed and two-level anterior cervical fusion was performed.
Kim et al[24] described the outcomes of ABL in three patients. The average distance of ABL was 2.57 mm (range 2.0-3.0 mm). These three patients achieved satisfying clinical outcomes without any change in symptoms during follow-up, and their pre- and post-operative read only memory were in the average range of this study. Ossification of the anterior longitudinal ligament (ALL) at the inferior adjacent level was noted in one patient at the two-year follow-up.
Heo et al[25] addressed the clinical effects of ABL. In their research, patients in the major change group (implant exposure) suffered from obvious neck pain postoperatively. The visual analogue scores for neck pain at 6 mo and 12 mo after surgery were 5.3 ± 2.0 and 4.7 ± 1.7, respectively, in the major change group; and 1.8 ± 1.1 and 1.8 ± 0.9, respectively, in the minor change group. Statistical differences were found between the two groups. The motion function was preserved at the last follow-up in the patients with ABL.
Kieser et al[26,27] reported that ABL caused no statistical difference in visual analogue score for arm or neck pain at any time after surgery, although the neck disability index score showed a decreasing trend at the last follow-up in patients with ABL (18.9 in the non-ABL group, 11.2 in the mild group, 10.1 in the moderate group, and 9.0 in the severe group, P = 0.094). Implant exposure or subsidence was observed in patients with severe ABL. Additionally, a significant relationship between Grade 4 HO and ABL was found in their research.
Kieser et al[26,27] analyzed the potential risk factors for ABL. In their retrospective studies with a minimum follow-up of five years, they found that the number of levels operated was the only risk factor. Other variables including age, sex, cervical alignment, read only memory, and implant position were not related to ABL. They hypothesized that the extensive exposure and drilling of vertebra might cause a direct osteolytic insult, and the more the dissection and soft-tissue stripping during multilevel surgery, the greater the bone insult. In addition, Kieser found that the cranial endplate was exposed to a greater risk of ABL, which could be due to resection of the ALL. The ALL attaches tightly to the inferior portion of the ventral surface of the cervical vertebrae, and this region would be non-loaded and subsequently resorbed after resection of the ALL.
In 2019, Kieser et al[27] proposed the mechanism of ABL of different degrees. The direct heat necrosis of the vertebra and resection of the ALL may cause Grade 1-2 ABL. Injury to nutrient vessels of the vertebra and subsequent avascular necrosis may lead to grade 3-4 ABL.
Heo et al[25] considered that the stress shielding effects played an important role in ABL. They observed a complete cortical bone margin of the ABL area based on computed tomography scans, and did not find any radiolucent osteolytic lesion adjacent to the implants. This opinion was consistent with that of Kim et al[24].
Kim et al[24] also speculated that the wear debris of artificial discs might induce the inflammatory response, and the inflammatory factors would recruit pre-osteoclasts and initiate the bone remodeling process.
Hacker et al[23] believed that low virulence bacterial infection of the endplate may account for the ABL in their case. They evaluated the tissue samples around the implant and did not find any pathological agent or culture except for macrophages.
In contrast, Ren et al[22] thought that ABL did not look like a low-grade infection. They hypothesized that micromovement would cause mechanical damage to the adjacent vertebra and subsequent bone loss.
Peri-implant bone loss has been widely reported in large joint arthroplasty[29-31]. However, studies on bone loss after CDR are lacking. By reviewing the literature, we classified the mechanism of ABL into three major hypotheses: stress shielding, micromovement, and injury to nutrient vessels.
The stress shielding effect has been documented as one of the factors of bone loss in hip and knee replacement[29]. In CDR, this term may be more accurately named as stress concentration. Physiologically, the axial loads transfer evenly and gently from one vertebra to the intervertebral disc, then to another vertebra. However, this mechanical system is altered after disc arthroplasty; the axial loads would transfer through the implant. Therefore, the stress would concentrate on where the implant is located. On the one hand, some artificial discs show obvious stress concentration in the anterior part of the endplates[32,33]. The hyper-pressure in the anterior region can activate the process of bone resorption[34]. On the other hand, the artificial disc does not cover the endplate sufficiently[35,36]. For the non-keel designed artificial disc, for instance, Discocerv and Baguera-C, the anterior part of the endplates cannot be covered, which may lead to hypo-pressure in this region. Based on Wolff’s law, the anterior vertebral hypo-pressure from the artificial disc would initiate bone resorption. Consistent with this hypothesis, previous finite element studies showed that stress was distributed in the covered area of the endplates at the surgical level[37,38]. In addition, Wang et al[21] noted that patients with ABL showed a significantly more lordotic disc angle (which may shift the axial load posteriorly), compared with those who developed anterior HO. Accordingly, stress concentration may be one major reason for the development of ABL.
Micromovement of the implant into the vertebra may induce mechanical damage to the peri-implant bone and subsequent bone loss[22,23]. On the other hand, micromotion may cause artificial discs to produce friction and wear debris, which may induce an inflammatory response and peri-implant bone loss[29,39,40]. Basic studies have proved that the wear debris can initiate bone loss via the RANK/RANKL/OPG pathway, and the inhibition of inflammation or RANK/RANKL pathway has protective effects on peri-implant osteolysis[41-43]. In addition, debris can also induce innervation and pain factor production[42].
Although wear debris-induced osteolysis is widely accepted in large joint arthroplasty, this hypothesis is flawed with regard to two aspects in CDR. First, bone loss caused by wear debris is not confined to the anterior portion of the vertebra, but is around the surface of the implant. For instance, Devin et al[44] reported wear debris-induced bone loss after lumbar disc replacement. Massive osteolysis was noted in the central and posterior region of the vertebral body, and debris was scattered in the adjacent bones. Veruva et al[45] described the presence of osteolytic cysts distributed around artificial lumbar discs caused by the debris. These findings suggest that wear debris-induced bone loss is different to ABL in phenotype. Second, ABL was observed within 3 mo in most cases, and had a self-limited course. In contrast, debris-induced osteolysis may be evident on radiographs after a longer time and may be progressive, which was reported by Tumialán et al[46]. They described bone loss which was noted 9 mo after Prodisc-C implantation. The osteolytic process did not stop until the implant was removed. To sum up, wear debris is not a likely cause of ABL.
The third major hypothesis of ABL is the injury to nutrient vessels of the anterior vertebrae. The anatomic study by Dunbar et al[47] demonstrated the abundant nutrient blood supply in the anterior part of the cervical spine[48]. These vessels pass over the longus colli, and penetrate vertebrae mainly on the anterior surface of the cervical vertebrae. Usually, these nutrient vessels are coagulated to reduce intraoperative bleeding, and this may cause avascular necrosis of the anterior part of the endplate. The anterior nutrient foraminae are mainly located in the superior third of the vertebrae, leaving the inferior portion hypo-vascular. Therefore, theoretically, the inferior portion of vertebrae would be at a higher risk of avascular necrosis, and this is consistent with the clinical findings of Kieser et al[27]. Consequently, ABL may be related to avascular necrosis caused by injury to nutrient vessels.
Kieser et al[26] showed that the more levels operated, the higher the risk of developing ABL. A possible explanation for this might be that multilevel surgery would change the biomechanical properties of the cervical spine leading to hyper- or hypo-pressure in the anterior region of the vertebrae, as described earlier. Additionally, we believe the design of artificial discs is another risk factor in the development of ABL. The prevalence of ABL was different among artificial discs, and this could be attributed to the discrepancy in stress distribution of the endplates of different types of artificial discs.
Some authors consider that heat necrosis due to the burring and milling process could inactivate osteocytes of the anterior vertebra, and result in a direct osteolytic insult. However, the findings of Heo et al[25] and Kieser et al[26,27] do not support this assumption. Even though the anterior cortex of the vertebra was carefully preserved during surgery, ABL still occurred. In addition, Wang et al[21] found that the anterior milling ratio and milling angle were comparable between patients with ABL or anterior bone formation. Therefore, heat necrosis may not be the most important risk factor for ABL.
Usually, patients with ABL do not have clear clinical and radiological effects, while severe ABL may produce segmental kyphosis and local pain[23-25]. Due to the self-limited process, most cases do not require intervention. However, we hold the view that early interventions including rehabilitation exercise and pain relief are necessary for these patients. In our center, we have seen patients with ABL complain of persistent neck pain for one year after surgery. Early interventions can improve the satisfaction of surgery and quality of life. In addition, the impacts of ABL on the adjacent segments remain unknown. Further studies with long-term follow-up are needed to clarify the relationship between ABL and adjacent segment degeneration.
Currently, ABL is observed on lateral radiographs. Notwithstanding this method is simple and easy, but it may increase the false positive rate of ABL. Occasionally, the inappropriate irradiation direction of X-rays may give us a false impression that ABL has occurred. For this reason, computed tomography scans are better in evaluating the prevalence of ABL[25]. Moreover, to prevent and identify ABL early, further studies on the mechanism and risk factors of ABL after CDR are needed.
There are several limitations in our study. The major limitation is the small sample size of each included study. Studies with large sample size are needed to determine the precise prevalence of ABL. As diverse evaluation systems for ABL were used in the included studies, it is difficult to make accurate assessments on the impacts of ABL. In addition, most studies only used non-keeled artificial discs; thus, ABL in keeled artificial discs, such as Prodisc-C and Prestige-LP, is unknown. Finally, we focused on prospective and retrospective studies, and may have missed a wider discussion on the underlying mechanism of ABL in some case reports or case series. Further studies are required to elucidate these limitations.
ABL is common in CDR. ABL occurs within 3-6 mo, and stops 12 mo after surgery. Several cases were noted to have a self-healing process. ABL does not have obvious clinical or radiological effects in most patients, while severe ABL may result in segmental kyphosis and persistent pain. ABL may be related to HO; however further studies are required to confirm this. Multilevel surgery is recognized as a potential risk factor for ABL, and the underlying mechanisms include stress concentration and injury to nutrient vessels. ABL should be considered a complication after CDR as it was associated with neck pain, implant subsidence, and HO.
Anterior bone loss (ABL) after cervical disc replacement (CDR) has attracted considerable concern in recent years. Whether ABL is a radiological anomaly or a complication remains unknown.
Several studies have reported the prevalence, impacts, and outcomes of ABL. However, an overall understanding of ABL is lacking.
This study aimed to comprehensively evaluate ABL after CDR.
A systematic review was performed according to the preferred reporting items for systematic reviews guideline.
The prevalence of ABL ranges from 3.13% to 91.89%, and multilevel surgery may be one of the risk factors for ABL. ABL occurred within 6 mo postoperatively and stopped after 1 year. Severe cases may result in kyphosis, implant subsidence, and neck pain.
ABL is a common condition after CDR. ABL should be considered a complication after CDR due to its clinical impacts.
Further studies should clarify the relationship between ABL and adjacent segment degeneration. Further studies on the mechanism and risk factors for ABL are needed. The method used to measure ABL should be improved.
We wish to thank Dr. Ying-Jun Guo and Dr. Jun-Bo He for advice on the discussion.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mehren C S-Editor: Zhang L L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Liu Y, Qi M, Chen H, Yang L, Wang X, Shi G, Gao R, Wang C, Yuan W. Comparative analysis of complications of different reconstructive techniques following anterior decompression for multilevel cervical spondylotic myelopathy. Eur Spine J. 2012;21:2428-2435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Lee DH, Cho JH, Hwang CJ, Lee CS, Cho SK, Kim C, Ha JK. What Is the Fate of Pseudarthrosis Detected 1 Year After Anterior Cervical Discectomy and Fusion? Spine (Phila Pa 1976). 2018;43:E23-E28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Buttermann GR. Anterior Cervical Discectomy and Fusion Outcomes over 10 Years: A Prospective Study. Spine (Phila Pa 1976). 2018;43:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Dong L, Xu Z, Chen X, Wang D, Li D, Liu T, Hao D. The change of adjacent segment after cervical disc arthroplasty compared with anterior cervical discectomy and fusion: a meta-analysis of randomized controlled trials. Spine J. 2017;17:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Jaumard NV, Bauman JA, Guarino BB, Gokhale AJ, Lipschutz DE, Weisshaar CL, Welch WC, Winkelstein BA. ProDisc cervical arthroplasty does not alter facet joint contact pressure during lateral bending or axial torsion. Spine (Phila Pa 1976). 2013;38:E84-E93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Galbusera F, Bellini CM, Brayda-Bruno M, Fornari M. Biomechanical studies on cervical total disc arthroplasty: a literature review. Clin Biomech (Bristol, Avon). 2008;23:1095-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Kelly MP, Eliasberg CD, Riley MS, Ajiboye RM, SooHoo NF. Reoperation and complications after anterior cervical discectomy and fusion and cervical disc arthroplasty: a study of 52,395 cases. Eur Spine J. 2018;27:1432-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Gutman G, Rosenzweig DH, Golan JD. Surgical Treatment of Cervical Radiculopathy: Meta-analysis of Randomized Controlled Trials. Spine (Phila Pa 1976). 2018;43:E365-E372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Kim JS, Dowdell J, Cheung ZB, Arvind V, Sun L, Jandhyala C, Ukogu C, Ranson W, Jacobs S, McAnany S, Cho SK. The Seven-Year Cost-Effectiveness of Anterior Cervical Discectomy and Fusion Versus Cervical Disc Arthroplasty: A Markov Analysis. Spine (Phila Pa 1976). 2018;43:1543-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Qureshi S, Goz V, McAnany S, Cho SK, Hecht AC, Delamarter RB, Fehlings MG. Health state utility of patients with single-level cervical degenerative disc disease: comparison of anterior cervical discectomy and fusion with cervical disc arthroplasty. J Neurosurg Spine. 2014;20:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Yang SD, Zhu YB, Yan SZ, Di J, Yang DL, Ding WY. Anterior cervical discectomy and fusion surgery versus total disc replacement: A comparative study with minimum of 10-year follow-up. Sci Rep. 2017;7:16443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mehren C, Heider F, Siepe CJ, Zillner B, Kothe R, Korge A, Mayer HM. Clinical and radiological outcome at 10 years of follow-up after total cervical disc replacement. Eur Spine J. 2017;26:2441-2449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Gornet MF, Burkus JK, Shaffrey ME, Schranck FW, Copay AG. Cervical disc arthroplasty: 10-year outcomes of the Prestige LP cervical disc at a single level. J Neurosurg Spine. 2019;31:317-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Lavelle WF, Riew KD, Levi AD, Florman JE. Ten-year Outcomes of Cervical Disc Replacement With the BRYAN Cervical Disc: Results From a Prospective, Randomized, Controlled Clinical Trial. Spine (Phila Pa 1976). 2019;44:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Gornet MF, Lanman TH, Burkus JK, Dryer RF, McConnell JR, Hodges SD, Schranck FW. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine. 2019;1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Ozbek Z, Ozkara E, Arslantaş A. Implant Migration in Cervical Disk Arthroplasty. World Neurosurg. 2017;97:390-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Zhou F, Ju KL, Zhao Y, Zhang F, Pan S, Heller JG, Sun Y. Progressive Bone Formation After Cervical Disc Replacement: Minimum of 5-Year Follow-up. Spine (Phila Pa 1976). 2018;43:E163-E170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Nunley PD, Cavanaugh DA, Kerr EJ, Utter PA, Campbell PG, Frank KA, Marshall KE, Stone MB. Heterotopic Ossification After Cervical Total Disc Replacement at 7 Years-Prevalence, Progression, Clinical Implications, and Risk Factors. Int J Spine Surg. 2018;12:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Li G, Wang Q, Liu H, Yang Y. Postoperative Heterotopic Ossification After Cervical Disc Replacement Is Likely a Reflection of the Degeneration Process. World Neurosurg. 2019;125:e1063-e1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Zeng J, Liu H, Chen H, Rong X, Meng Y, Yang Y, Deng Y, Ding C. Effect of Prosthesis Width and Depth on Heterotopic Ossification After Cervical Disc Arthroplasty. Spine (Phila Pa 1976). 2019;44:624-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Wang X, Meng Y, Liu H, Hong Y, Wang B. Is Anterior Bone Loss the Opposite of Anterior Heterotopic Ossification in Prestige-LP Cervical Disc Replacement? World Neurosurg. 2020;136:e407-e418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Ren X, Wang W, Chu T, Wang J, Li C, Jiang T. The intermediate clinical outcome and its limitations of Bryan cervical arthroplasty for treatment of cervical disc herniation. J Spinal Disord Tech. 2011;24:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Hacker FM, Babcock RM, Hacker RJ. Very late complications of cervical arthroplasty: results of 2 controlled randomized prospective studies from a single investigator site. Spine (Phila Pa 1976). 2013;38:2223-2226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Kim SH, Chung YS, Ropper AE, Min KH, Ahn TK, Won KS, Shin DA, Han IB. Bone loss of the superior adjacent vertebral body immediately posterior to the anterior flange of Bryan cervical disc. Eur Spine J. 2015;24:2872-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Heo DH, Lee DC, Oh JY, Park CK. Bone loss of vertebral bodies at the operative segment after cervical arthroplasty: a potential complication? Neurosurg Focus. 2017;42:E7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Kieser DC, Cawley DT, Fujishiro T, Mazas S, Boissière L, Obeid I, Pointillart V, Vital JM, Gille O. Risk factors for anterior bone loss in cervical disc arthroplasty. J Neurosurg Spine. 2018;29:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kieser DC, Cawley DT, Fujishiro T, Tavolaro C, Mazas S, Boissiere L, Obeid I, Pointillart V, Vital JM, Gille O. Anterior Bone Loss in Cervical Disc Arthroplasty. Asian Spine J. 2019;13:13-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | CEBM. ‘The Oxford Levels of Evidence 2’. Oxford Cent Evidence-Based Med [Internet] 2016. Available from: http://www.cebm.net/index.aspx?o=5653. [Cited in This Article: ] |
| 29. | Gallo J, Goodman SB, Konttinen YT, Wimmer MA, Holinka M. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater. 2013;9:8046-8058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;124-134. [PubMed] [Cited in This Article: ] |
| 31. | Dalat F, Barnoud R, Fessy MH, Besse JL, French Association of Foot Surgery AFCP. Histologic study of periprosthetic osteolytic lesions after AES total ankle replacement. A 22 case series. Orthop Traumatol Surg Res. 2013;99:S285-S295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Mo Z, Zhao Y, Du C, Sun Y, Zhang M, Fan Y. Does location of rotation center in artificial disc affect cervical biomechanics? Spine (Phila Pa 1976). 2015;40:E469-E475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Mo Z, Li Q, Jia Z, Yang J, Wong DW, Fan Y. Biomechanical consideration of prosthesis selection in hybrid surgery for bi-level cervical disc degenerative diseases. Eur Spine J. 2017;26:1181-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001;262:398-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Dong L, Tan MS, Yan QH, Yi P, Yang F, Tang XS, Hao QY. Footprint mismatch of cervical disc prostheses with Chinese cervical anatomic dimensions. Chin Med J (Engl). 2015;128:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Thaler M, Hartmann S, Gstöttner M, Lechner R, Gabl M, Bach C. Footprint mismatch in total cervical disc arthroplasty. Eur Spine J. 2013;22:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Lin CY, Kang H, Rouleau JP, Hollister SJ, Marca FL. Stress analysis of the interface between cervical vertebrae end plates and the Bryan, Prestige LP, and ProDisc-C cervical disc prostheses: an in vivo image-based finite element study. Spine (Phila Pa 1976). 2009;34:1554-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Yu CC, Liu P, Huang DG, Jiang YH, Feng H, Hao DJ. A new cervical artificial disc prosthesis based on physiological curvature of end plate: a finite element analysis. Spine J. 2016;16:1384-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Hallab NJ, Cunningham BW, Jacobs JJ. Spinal implant debris-induced osteolysis. Spine (Phila Pa 1976). 2003;28:S125-S138. [PubMed] [Cited in This Article: ] |
| 40. | Veruva SY, Lanman TH, Isaza JE, Freeman TA, Kurtz SM, Steinbeck MJ. Periprosthetic UHMWPE Wear Debris Induces Inflammation, Vascularization, and Innervation After Total Disc Replacement in the Lumbar Spine. Clin Orthop Relat Res. 2017;475:1369-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Hartmann ES, Köhler MI, Huber F, Redeker JI, Schmitt B, Schmitt-Sody M, Summer B, Fottner A, Jansson V, Mayer-Wagner S. Factors regulating bone remodeling processes in aseptic implant loosening. J Orthop Res. 2017;35:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Punt IM, Cleutjens JP, de Bruin T, Willems PC, Kurtz SM, van Rhijn LW, Schurink GW, van Ooij A. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009;30:2079-2084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Córdova LA, Trichet V, Escriou V, Rosset P, Amiaud J, Battaglia S, Charrier C, Berreur M, Brion R, Gouin F, Layrolle P, Passuti N, Heymann D. Inhibition of osteolysis and increase of bone formation after local administration of siRNA-targeting RANK in a polyethylene particle-induced osteolysis model. Acta Biomater. 2015;13:150-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Devin CJ, Myers TG, Kang JD. Chronic failure of a lumbar total disc replacement with osteolysis. Report of a case with nineteen-year follow-up. J Bone Joint Surg Am. 2008;90:2230-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Veruva SY, Lanman TH, Hanzlik JA, Kurtz SM, Steinbeck MJ. Rare complications of osteolysis and periprosthetic tissue reactions after hybrid and non-hybrid total disc replacement. Eur Spine J. 2015;24 Suppl 4:S494-S501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Tumialán LM, Gluf WM. Progressive vertebral body osteolysis after cervical disc arthroplasty. Spine (Phila Pa 1976). 2011;36:E973-E978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Dunbar L, Vidakovic H, Löffler S, Hammer N, Gille O, Boissiere L, Obeid I, Pointillart V, Vital JM, Kieser DC. Anterior cervical spine blood supply: a cadaveric study. Surg Radiol Anat. 2019;41:607-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Harris RS, Jones DM. The arterial supply to the adult cervical vertebral bodies. J Bone Joint Surg Br. 1956;38-B:922-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.2] [Reference Citation Analysis (0)] |