Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.353
Peer-review started: August 30, 2019
First decision: November 9, 2019
Revised: December 11, 2019
Accepted: December 21, 2019
Article in press: December 21, 2019
Published online: January 26, 2020
Esophageal bronchogenic cyst (EBC) is a rare congenital disease that is difficult to diagnose preoperatively, and treatment remains controversial.
We report a 53-year-old Chinese woman hospitalized in our hospital following the discovery of a submucosal protruding mass of the esophagus by upper endoscopy. A preliminary diagnosis of EBC was made by endoscopic ultrasonography (EUS), and treatment was accomplished by endoscopic submucosal tunnel dissection (ESTD). The pathological results verified the diagnosis. No scar changes or cystic lesion within the original lesion were found under EUS after a 3-mo follow-up.
EUS is valuable for the preliminary diagnosis of EBC and surveillance. ESTD is a safe and effective treatment for EBC. Further evaluation of complications and long-term follow-ups are required.
Core tip: Bronchogenic cyst is a rare congenital lesion that arises from malformation of the primitive foregut. Esophageal bronchogenic cyst (EBC) is uncommon and is rarely reported in recent literature, with difficult preoperative diagnosis and controversial treatment. We report a patient with EBC. Endoscopic ultrasonography can serve as a valuable tool for preliminary diagnosis, differential diagnosis and surveillance. Endoscopic submucosal tunneling dissection is a safe and effective method for the treatment of EBCs.
- Citation: Zhang FM, Chen HT, Ning LG, Xu Y, Xu GQ. Esophageal bronchogenic cyst excised by endoscopic submucosal tunnel dissection: A case report. World J Clin Cases 2020; 8(2): 353-361
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/353.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.353
Bronchogenic cyst is a rare congenital lesion that arises from malformation during the fifth to eighth week of gestation when the primitive foregut divides into the ventral trachea[1]. It occurs mostly in the middle and superior mediastinum, and account for 10-15% of all primary masses of the mediastinum, while the esophageal type (esophageal bronchogenic cyst, EBC) is even more uncommon and have only been reported in 23 adult cases since 1981[1,2]. In spite of improved imaging techniques, a definitive preoperative diagnosis and differential diagnosis between bronchogenic cyst and esophageal leiomyoma, duplication cyst, lymphadenopathy and pleural fibroma may still be difficult to establish[3]. The management of EBC remains controversial, in consideration of the possible complication of infection, intracystic hemorrhage, rupture, and carcinomatous changes. Complete surgical resection may be appropriate, but may not be recommended for small cysts found in asymptomatic patients[4,5]. The recurrence time of EBC after incomplete resection is reported to be > 20 years[6]; thus, thoracoscopic resection has been selected as a less invasive alternative. If the EBC is located in the esophageal wall, mucosal or submucosal resection is confirmed to be safe, and endoscopic resection may be an effective and less invasive treatment method for diagnosis and treatment compared with thoracoscopy or surgery[7,8].
Here, we report a case of EBC which was preliminary diagnosed by endoscopic ultrasonography (EUS) and pathologically confirmed by endoscopic submucosal tunnel dissection (ESTD). This suggests that if accessibility and safety are confirmed, ESTD might be a less invasive and less complicated approach that is effective for diagnosis and treatment. The timeline is shown in Table 1.
| Time | Information about the patient |
| March 15, 2019 | Admitted to our hospital |
| March 15-19, 2019 | Received laboratory and imaging examinations |
| March 19, 2019 | Received EUS |
| March 21, 2019 | Underwent ESTD |
| March 26, 2019 | Discharged from hospital |
| March 27, 2019 | Pathological results confirmed EBC |
| June 20, 2019 | Follow-up and gastroscopy re-examination |
A 53-year-old Chinese woman had health checkups, including gastroscopy examinations, and then was hospitalized in our hospital following the finding of a submucosal lesion in upper endoscopy. She had no complaint of dysphagia, odynophagia, abdominal pain, poor appetite or weight loss.
Gastroscopy performed in another hospital showed a submucosal protruding mass in the esophagus 25 cm from the incisors, and a diagnosis of external pressure esophageal apophysis was considered.
The patient had a history of hypertension for more than 2 years, and was treated daily with oral antihypertensive drugs in the form of 2.5 mg levamlodipine dispersible tablets.
The patient’s family history and past medical history were both unremarkable. She had no history of esophagitis or esophageal tuberculosis.
The patient’s temperature was 36.8°C, heart rate was 80 bpm, respiratory rate was 19 breaths/min and blood pressure was 125/80 mmHg. The breath sounds of both lungs were clear, no dry and wet rales were heard, the abdomen was soft, with no tenderness, no rebound pain and no palpable mass.
During hospitalization, routine laboratory parameters were within the normal range. Tumor markers including carcinoembryonic antigen and cancer antigen 125 were negative.
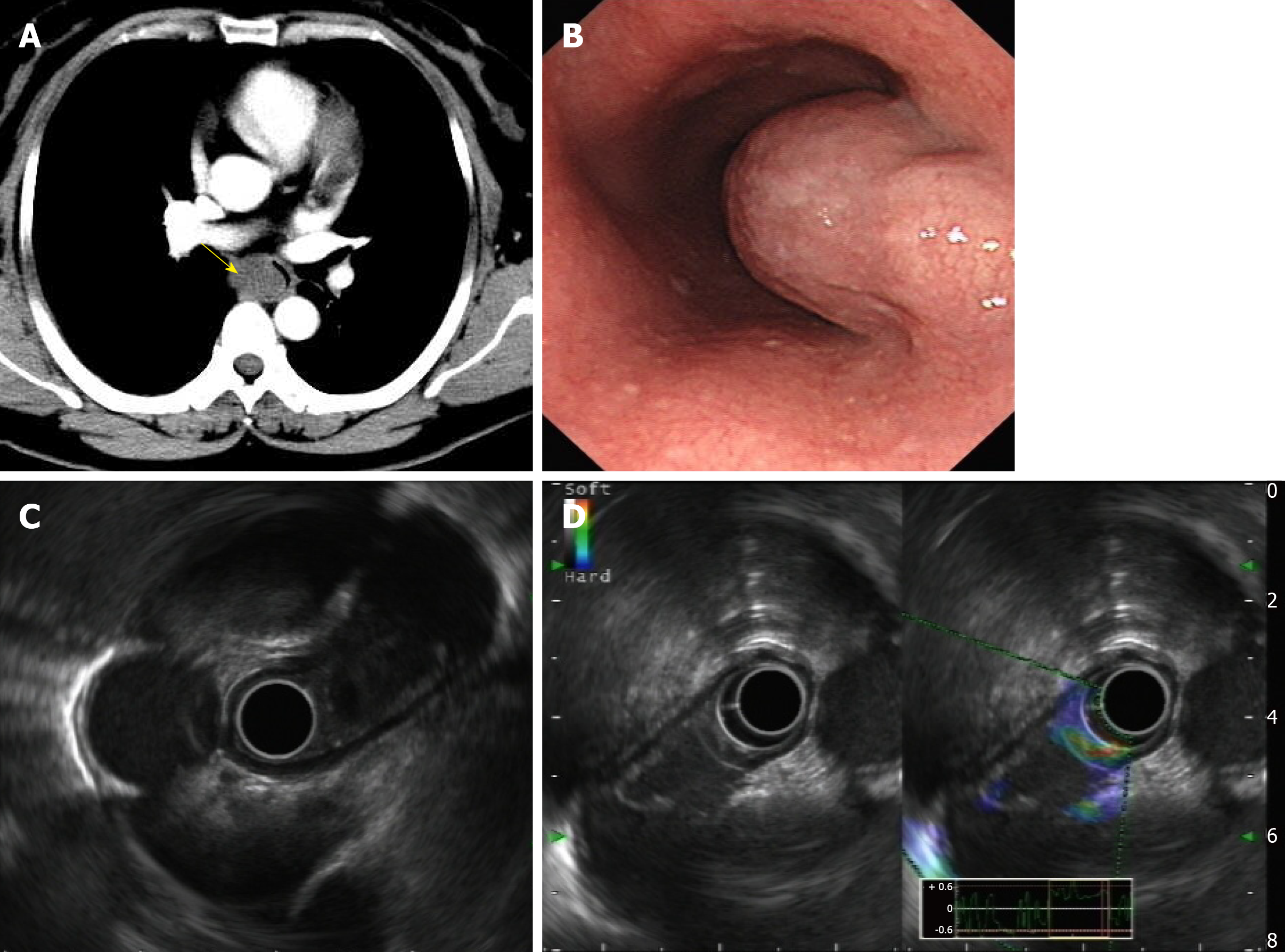
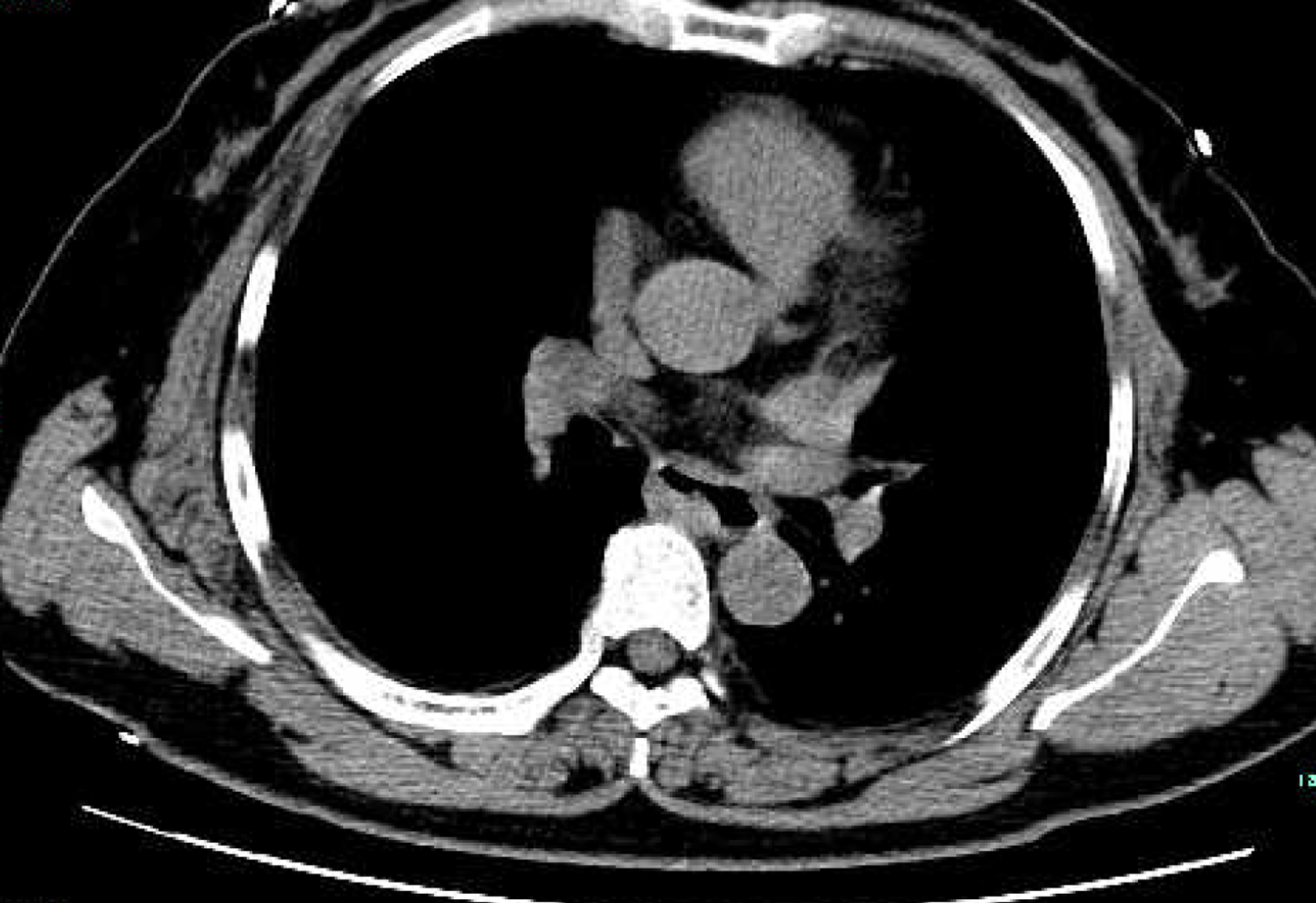
In order to help reveal the nature of a cyst, enhanced thoracic computed tomography (CT) was done, and it revealed an ovular low-density shadow with a clear boundary of about 3.6 cm × 1.8 cm in the upper middle part of the esophagus, with mild to moderate enhancement. There was no obvious thickening of the esophageal wall, no obvious dilatation or obstruction of the esophagus, and no obvious enlarged lymph nodes in the mediastinum (Figure 1A). Thus, a diagnosis of esophageal leiomyoma was considered.
Upon upper gastrointestinal endoscopy, a submucosal mass was observed about 28 cm from the incisor with a gourd-like appearance, and the size was about 4.0 cm × 2.0 cm (Figure 1B).
EUS with a 12-MHz radial probe revealed a hypoechoic irregular mass arising within the propria muscularis in the middle segment of the esophagus (28 cm from the incisor), with a clear boundary, cystic wall, uneven echo, spot-like echo, and a separation zone inside. The measured area was 3.2 cm × 2.0 cm. Contrast-enhanced US showed enhancement around the lesion but no internal enhancement (Figure 1C and D).
EUS showed a hypoechoic irregular cystic-solid mass originating from the muscularis propria, with a cystic wall and clear boundary, a nonuniform spotty internal echo and internal partition zone. Contrast-enhanced US showed slight enhancement around the lesion but no enhancement inside. Thus, vascular lesions such as hemangioma, solitary varix or other vascularized cystic lesions were excluded. According to the above features, a preliminary diagnosis of EBC was made.
With respect to the treatment of EBC in asymptomatic patients, opinions are divided. Some researchers advocate that the least invasive method should be chosen for diagnosis and treatment of EBC due to its low rate of malignant transformation. Others recommend surgical or thoracoscopic removal in consideration of complications of intracystic hemorrhage, rupture, infection and carcinomatous changes. Surveillance and resection options were discussed with the patient and her family. Esophagectomy, thoracoscopic resection, and ESTD were considered as possible treatments, which were communicated to them. They wanted a definite diagnosis, as the lesion was small and originated from the muscularis propria. Preoperative endoscopy was not difficult and caused little damage to the mucosa and muscular layer, and in accordance with the principles of endoscopic treatment of esophageal cancer in the muscularis propria, ESTD was recommended. If the pathological results show a benign lesion, long-term follow-up surveillance is still required, and prognosis and complications should also be further assessed. EUS seemed to be an effective and valuable option. If the pathological results show a malignant lesion, supplementary surgery is suggested. The patient ultimately opted for ESTD, for which she gave informed consent.
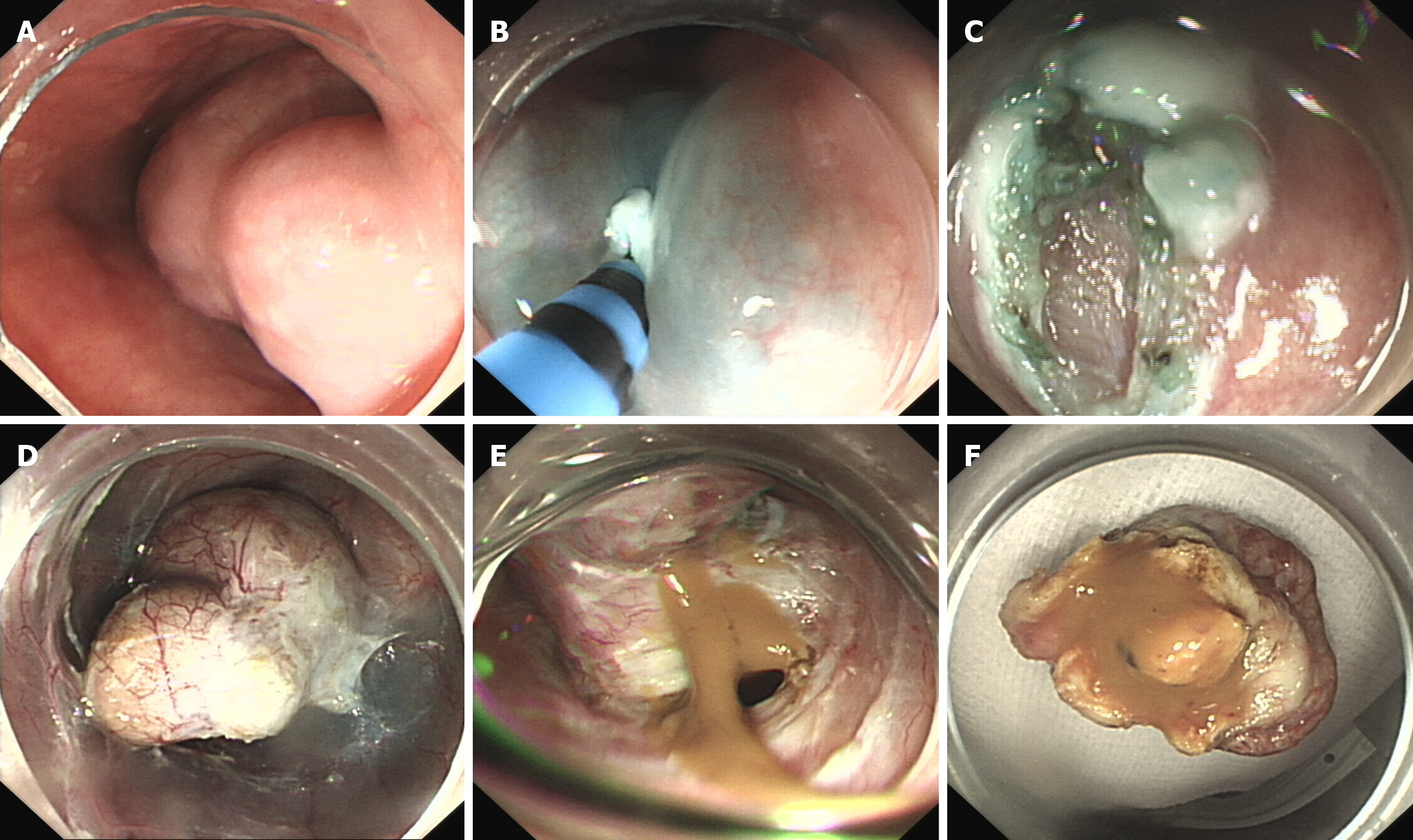
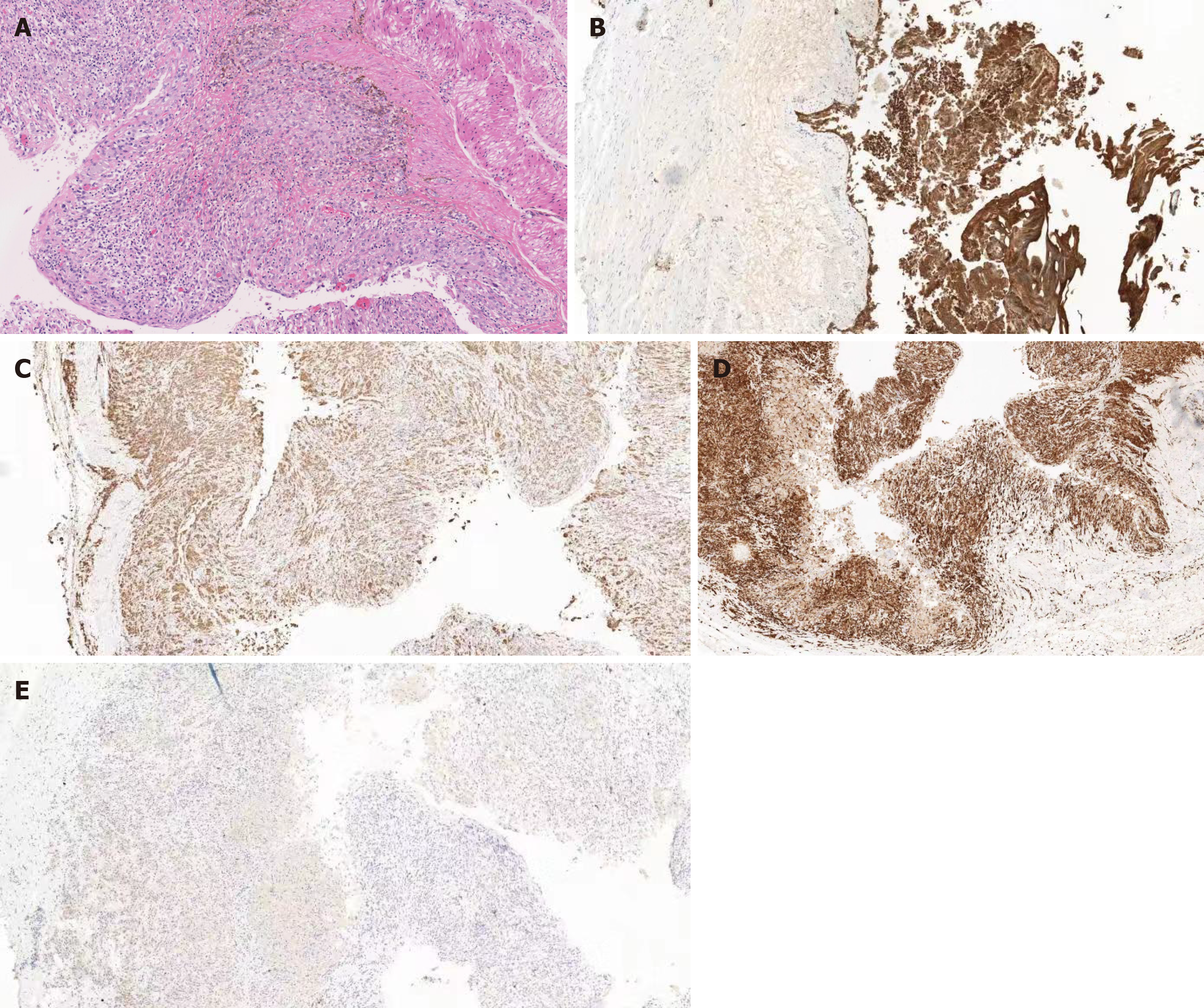
A submucosal mass was observed about 28 cm from the incisor with a gourd-like appearance, and the size was about 4.0 cm × 2.0 cm. Submucosal injection of normal saline with adrenaline and indigo carmine solution (1:10000) at 5 cm of the mouth side from the lesion was given first. Then, HybridKnife that can complete marking, cutting, hemostasis and other endoscopic resection steps was used to precut 2 cm of the esophageal mucosa. Submucosal separation was then performed to create a tunnel until the tumor appeared. The tumor was located in the muscular layer, and after the muscular tissue surrounding the tumor was separated, the tumor was completely exposed. Tumor was yellow-white and was soft to the touch. Then, HybridKnife was continually used for stripping. After most of the lesion was dissected, a small defect in the basilar cyst wall appeared, with yellow gelatinous liquid flowing out of the breach. The liquid was suctioned and the tumor was removed after continual stripping, and was then sent for further pathological testing. The tunnel was repeatedly washed with normal saline, and endoclips were used to close the entrance to the submucosal tunnel (Figure 2). A histopathological examination revealed a respiratory ciliated columnar epithelium-lined cyst wall containing smooth muscle, vasculature and cartilage (Figure 3). An EBC was diagnosed.
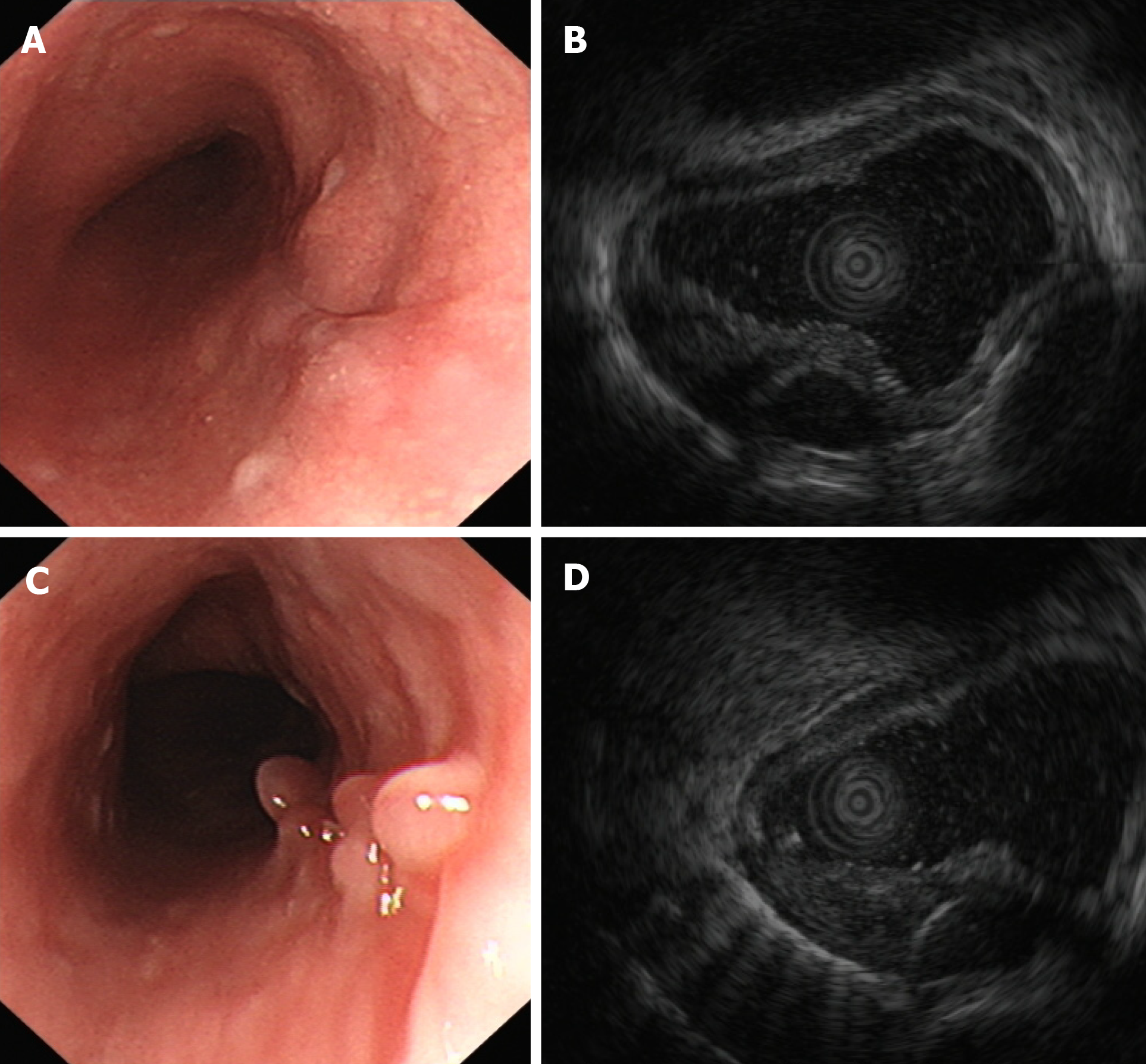
After the operation, the patient was fasted for 2 d, rehydrated, and given both acid inhibition and anti-infective treatment. The patient was discharged 5 d later and given oral mucosal protectant (teprenone, 50 mg tid) for one month. During follow-up, the patient remained asymptomatic and had no adverse effects from the mucosal protectant. Gastroscopic re-examination 5 mo after surgery showed scar changes after endoscopic excision 21-25 cm from the incisors of the esophagus, and granular mucous membrane uplifts with a smooth surface were observed 26 cm from the incisors of the esophagus (Figure 4A and B). No cystic lesion was found within the original lesion under EUS, and local hypoechoic thickening of the intrinsic muscle layer was found at a size of 5.2 mm × 9.1 mm. The hierarchical structure of the esophageal wall remained clear, and the surrounding esophageal wall appeared normal (Figure 4C and D). Esophageal CT plain scans showed no obvious thickening of the esophagus wall, no abnormal high-density shadows in the esophageal lumen, no obvious expansion of the esophagus, and no significantly enlarged lymph node shadows in the mediastinum (Figure 5).
EBC is a rare congenital disease, and most patients are female (65.2%) and middle-aged[2]. Its morbidity is ambiguous because most patients are asymptomatic[9]. If present, symptoms like dysphagia, cough, chest pain and dyspnea are caused by complications associated with the cyst itself or by oppression of the surrounding structures[10]. The patient in this case had no obvious discomfort and no abnormal signs.
Various imaging techniques can be used for diagnosis and differential diagnosis of EBC, esophageal leiomyoma, duplication cysts, lymphadenopathy and pleural fibroma[3]. Even so, making an explicit diagnosis remains difficult. Magnetic resonance imaging (MRI) or CT can sometimes reveal the nature of a cyst. Bronchogenic cysts typically present as spherical masses of either soft or water tissue attenuation in CT scans. When it manifests as water attenuation, differentiation from lymphadenopathy can be relatively easy[11]. However, when it presents as soft tissue attenuation, differentiation from solid lesions is relatively difficult[12].
In MRI, the typical appearance of a high-intensity lesion in both T1- and T2-weighted images can be seen due to the mucinous content. However, if EBC possesses high calcium content or protein or is infected, its density may be close to that of a solid mass, which increases diagnostic difficulty[13,14]. It is also difficult to identify the intramural and extramural relationship of EBC by CT or MRI. With ongoing technical improvements, diffusion-weighted MRI is used for distinguishing non-neoplastic cysts from solid masses in the mediastinum. This characterizes the mobility of water in the tissue, which can be less restricted in true cysts compared to any other solid masses with or without cystic degeneration. It also shows higher apparent diffusion coefficient values of non-neoplastic cysts than solid masses[15,16]. Unfortunately, we did not perform this advanced imaging.
In this case, the patient underwent many examinations including gastroscopy, CT and EUS. In another hospital, endoscopic examination indicated an external esophageal pressure eminence, and thoracic enhanced CT only suggested a middle and lower esophageal mass. Thoracic enhanced CT performed in our hospital suggested a diagnosis of esophageal leiomyoma. Finally, we made the primary diagnosis of EBC by EUS. EUS is valuable for distinguishing cystic lesions from solid masses due to its capacity to confirm the cystic nature and intra- and extramural extent of the lesion. Moreover, it is also sensitive for the diagnosis of intramural esophageal lesions, as it can clearly delineate the layer of origin as well as the relationship between the cyst and the esophagus[17].
Han et al[18] reviewed the literature on the EUS appearance of EBC in 2016, and only eight cases have been reported since 2000. They found that the cyst mostly originates from the muscularis propria; it can be ovular, round or tubular; and the appearance can range from hypoechoic to containing dense hyperechoic debris[18].
In the present case, EBC was preliminary diagnosed based on the typical EUS findings, which showed a hypoechoic irregular cystic-solid mass originating from the muscularis propria, with a cystic wall and clear boundary, a nonuniform spotty internal echo and internal partition zone. Contrast-enhanced US showed slight enhancement around the lesion but no enhancement inside. Thus, vascular lesions such as hemangioma, solitary varix or other vascularized cystic lesions were excluded.
EUS is a non-invasive technique that is easy operated and contributes to the diagnosis of EBC. However, EUS have some limitations; the primary diagnosis by EUS is related to the operator's level of experience. Besides, if EBC is atypical under EUS, as mentioned above, it may be misdiagnosed as other diseases.
EUS-fine-needle aspiration (EUS-FNA) is not advocated as a universal application. As for atypical and uncertain EBCs, EUS is also used to guide aspiration. EUS-FNA is recommended to obtain samples for cytological/histological diagnosis, and turbid paste contents aspirated from the tumor by EUS-FNA support the diagnosis of EBC[18,19]. However, using EUS-FNA for further diagnosis of EBC is not so easy because of the mucinous and viscous content of the cyst, as well as epithelial cells or debris, which lead to difficulty both in aspiration and cytological/histological diagnosis. In addition, in view of severe consequences of EUS-FNA, it is not routinely advocated in EBC[20].
With respect to the treatment of EBC in asymptomatic patients, opinions are divided. Some researchers advocate that the least invasive method should be chosen for diagnosis and treatment of EBC due to its low rate of malignant transformation. For example, EUS-FNA or biopsy by mediastinoscopy has been performed for diagnosis: endoscopic mucosal resection for removal of an EBC within the submucosal layer, endoscopic mucosal dissection for lesions located in the submucosal layer without involvement of the muscularis propria, and ESTD for EBC arising from the muscularis propria[8,21]. Others recommend surgical or thoracoscopic removal in consideration of complications of intracystic hemorrhage, rupture, infection and carcinomatous changes[4,5].
In our case, the lesion was small and located in the muscularis propria with uncertain preoperative diagnosis. Preoperative endoscopy was not difficult and caused little damage to the mucosa and muscular layer. The patient and family members insisted on endoscopic resection; therefore, ETSD was performed. However, for large and deep lesions, especially in the upper segment of the esophagus with obvious malignant tendencies, it is still necessary to choose surgical treatment for musculocutaneous tumors that cannot be completely treated under the microscope. These lesions are difficult to treat endoscopically once perforation and bleeding occur. Although endoscopic tunnel treatment of the EBC causes small local injury and has rapid recovery, it is still necessary to follow up and pay attention to recurrence, gastroesophageal reflux, esophageal diverticulum and stenosis, which are potential long-term complications. Our patient remained asymptomatic during follow-up and gastroscopy re-examination 5 mo after surgery, and showed scar changes after endoscopic excision. No cystic lesion was found within the original lesion, and local hypoechoic thickening of the intrinsic muscle layer was found under EUS. The hierarchical structure of the esophageal wall was still clear, and the surrounding esophageal wall was normal.
ESTD is a minimally invasive approach with few complications and hazards compared to surgery, especially for patients who are at high operative risk for a variety of factors[21,22]. If the ESTD specimen shows benign results histopathologically, long-term follow-up surveillance is still required, and prognosis and complications should also be further assessed. In this respect, EUS seems to be an effective and valuable option. If the ESTD specimen shows malignancy histopathologically, supplementary surgery is suggested.
For the approach to this case, there were some limitations. First, MRI, which can reveal the nature of a cyst, was not performed. However, if an EBC possesses high calcium or protein content, its density may be close to that of a solid mass, which increases diagnostic difficulty. Besides, it is also difficult to identify the intramural land extramural relationship of EBC by MRI. Thus, we did not perform MRI for the patient. Second, EUS-FNA was not recommended to obtain samples for ultimate cytological/histological diagnosis. This was because using EUS-FNA for further diagnosis of EBC is not easy due to the mucinous and viscous content of the cyst as well as epithelial cells or little debris, which lead to difficulty both in aspiration and cytological/histological diagnosis. In addition, in view of severe consequences of EUS-FNA and typical EUS findings, it was not advocated routinely. Third, long-term follow-up surveillance is still required, and prognosis and complications should also be further assessed. Until now, we have only assessed once 5 mo after ESTD. There are also some strengths, since the typical findings by EUS led us to make a primary diagnosis, and the histological examinations confirmed the diagnosis, indicating EUS was a noninvasive and useful tool for the preliminary diagnosis of EBC. Furthermore, the lesion was small and originated from the muscularis propria, preoperative endoscopic operation difficulty was relatively small with less damage to the mucosa and muscle layer, ESTD was recommended, and follow-up also suggested good results.
EBC is uncommon, with difficult preoperative diagnosis and controversial treatment. EUS is a valuable tool for preliminary diagnosis and surveillance. ESTD is a safe and effective method for treatment of EBC, although long term follow-up is essential.
Many thanks to Dan Ping Yu from the imaging department and Yan Feng Bai from the pathology department in our hospital for the interpretation of imaging results and pathological results.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kovacevic B, Shiryajev Y, Akbulut S, Dragoteanu MN, El-Razek AA, Hashimoto N S-Editor: Dou Y L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Thaller P, Blanchet C, Badr M, Mesnage R, Leboucq N, Mondain M, Cambonie G. Neonatal respiratory distress syndrome revealing a cervical bronchogenic cyst: a case report. BMC Pediatr. 2015;15:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Turkyilmaz A, Eroglu A, Subasi M, Findik G. Intramural esophageal bronchogenic cysts: a review of the literature. Dis Esophagus. 2007;20:461-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Chafik A, Benjelloun A, Qassif H, El Fikri A, El Barni R, Zrara I. Intramural esophageal bronchogenic cysts. Asian Cardiovasc Thorac Ann. 2011;19:69-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Chuang KH, Huang TW, Cheng YL, Chen JC, Tzao C, Chang H, Tsai WC, Lee SC. Esophageal bronchogenic cyst: a rare entity. Z Gastroenterol. 2007;45:958-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Hasegawa T, Murayama F, Endo S, Sohara Y. Recurrent bronchogenic cyst 15 years after incomplete excision. Interact Cardiovasc Thorac Surg. 2003;2:685-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108:880-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Bechara R, Onimaru M, Kushima M, Inoue H. Peroral endoscopic tumor resection for an esophageal bronchogenic cyst. Gastrointest Endosc. 2016;83:827-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Raptis A, Deprez PH, Jouret-Mourin A. Resection of an intra-esophageal bronchogenic cyst by endoscopic submucosal dissection. Dig Endosc. 2018;30:263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Liang JS, Yin GL, Zhang XM, Zhu SB, Dong YQ. Intramural esophageal bronchogenic cyst with wall hemorrhage results in acute esophageal obstruction: report of two cases. Am Surg. 2014;80:E27-E29. [PubMed] [Cited in This Article: ] |
| 10. | Altieri MS, Zheng R, Pryor AD, Heimann A, Ahn S, Telem DA. Esophageal bronchogenic cyst and review of the literature. Surg Endosc. 2015;29:3010-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217:441-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Ashizawa K, Okimoto T, Shirafuji T, Kusano H, Ayabe H, Hayashi K. Anterior mediastinal bronchogenic cyst: demonstration of complicating malignancy by CT and MRI. Br J Radiol. 2001;74:959-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pages ON, Rubin S, Baehrel B. Intra-esophageal rupture of a bronchogenic cyst. Interact Cardiovasc Thorac Surg. 2005;4:287-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Lim LL, Ho KY, Goh PM. Preoperative diagnosis of a paraesophageal bronchogenic cyst using endosonography. Ann Thorac Surg. 2002;73:633-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Abdel Razek AA, Gaballa G, Elashry R, Elkhamary S. Diffusion-weighted MR imaging of mediastinal lymphadenopathy in children. Jpn J Radiol. 2015;33:449-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Abdel Razek AA, Soliman N, Elashery R. Apparent diffusion coefficient values of mediastinal masses in children. Eur J Radiol. 2012;81:1311-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Cioffi U, de Simone M. Should video-assisted surgery be the first-line approach for bronchogenic cysts? Asian Cardiovasc Thorac Ann. 2011;19:289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Han C, Lin R, Yu J, Zhang Q, Zhang Y, Liu J, Ding Z, Hou X. A Case Report of Esophageal Bronchogenic Cyst and Review of the Literature With an Emphasis on Endoscopic Ultrasonography Appearance. Medicine (Baltimore). 2016;95:e3111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Sashiyama H, Miyazaki S, Okazaki Y, Kaiho T, Nakajima Y, Hoshino T, Akai T, Nabeya Y, Funami Y, Shimada H, Okazumi S, Ochiai T. Esophageal bronchogenic cyst successfully excised by endoscopic mucosal resection. Gastrointest Endosc. 2002;56:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Liu R, Adler DG. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound. 2014;3:152-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Tang X, Jiang B, Gong W. Endoscopic submucosal tunnel dissection of a bronchogenic esophageal cyst. Endoscopy. 2014;46 Suppl 1 UCTN:E626-E627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |