Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3349
Peer-review started: April 10, 2020
First decision: April 22, 2020
Revised: April 27, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 6, 2020
Alveolar soft part sarcoma (ASPS) is an extremely rare malignant sarcoma, accounting for less than 1% of all soft-tissue sarcomas. However, limited information is available on multimodal imaging [computed tomography (CT), magnetic resonance imaging (MRI), and positron emission computed tomography/computed tomography (PET/CT)] of ASPS.
This study reports a case of a 35-year-old female patient with ASPS of the left thigh with lung metastasis. The patient presented with a 1-year history of a palpable mass in the lower extremity, which exhibited rapid growth for 3 wk. CT, MRI, and F-deoxyglucose PET/CT examinations were performed. CT showed a slightly hypodense or isodense mass with patchy calcifications. On MRI examination, the mass manifested hyperintensity on T1-weighted, T2-weighted, and diffusion-weighted images with some signal voids. PET/CT images demonstrated an intensely hypermetabolic mass in the left thigh and hypermetabolic nodules in lungs.
ASPS should be considered as a possible diagnosis when a slow-growing mass is detected in the soft tissue of the extremities, with hyperintensity and numerous signal voids on T1-weighted, T2-weighted, and diffusion-weighted images and intense F-deoxyglucose uptake on PET/CT. ASPS can have calcifications on CT.
Core tip: Alveolar soft part sarcoma (ASPS) is a rare malignant soft-tissue tumor with a poor prognosis. ASPS have characteristic features on magnetic resonance imaging with hyperintensity and numerous signal voids on T1-weighted and T2-weighted images and intense F-deoxyglucose uptake on positron emission computed tomography/computed tomography images. Magnetic resonance imaging combined with positron emission computed tomography/computed tomography improve the differential diagnosis of ASPS. Calcification can be found on computed tomography images.
- Citation: Wu ZJ, Bian TT, Zhan XH, Dong C, Wang YL, Xu WJ. Computed tomography, magnetic resonance imaging, and F-deoxyglucose positron emission computed tomography/computed tomography findings of alveolar soft part sarcoma with calcification in the thigh: A case report. World J Clin Cases 2020; 8(15): 3349-3354
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3349.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3349
Alveolar soft part sarcoma (ASPS) is an extremely rare malignant sarcoma, constituting less than 1% of all soft-tissue sarcomas[1]. ASPS was first described by Christopherson et al[2] in 1952, deriving its name from its histological appearance[1-3]. The most common locations of ASPS are the lower extremities, head, and neck[4-6]. Metastases are usually demonstrated in 20%-40% of the patients at the time of diagnosis, commonly involving the lung, bone, and brain[7,8].
Several cases of ASPS have been reported that discuss the characteristics of magnetic resonance imaging (MRI) findings with hyperintensity on T1- and T2-weighted images and flow voids[8,9]. However, these changes were not specific and may mimic arteriovenous malformations or hemangioma. Various studies explored the manifestations of multimodal imaging examinations, especially diffusion-weighted imaging and positron emission computed tomography/computed tomography (PET/CT). The present study reports a case of a 35-year-old female patient with calcification in the mass found on multimodal imaging [computed tomography (CT), diffusion weighted imaging (DWI), conventional sequence MRI, and PET/CT], which was confirmed to be ASPS using core needle biopsy.
A 35-year-old woman complained of a palpable mass without pain in the left thigh for 1 year. The size of the mass increased suddenly, accompanied by pain starting from 3 wk ago.
The patient had no history of past illness and no family history of specific diseases.
The physical examination revealed a tender mass measuring 5.0 cm × 3.0 cm in the soft tissue of the left thigh without any obvious pulsation and local color change.
Results of routine laboratory examinations, including routine blood tests, blood biochemistry, routine urine tests and serum tumor markers, were within normal levels. There were no valuable laboratory data.
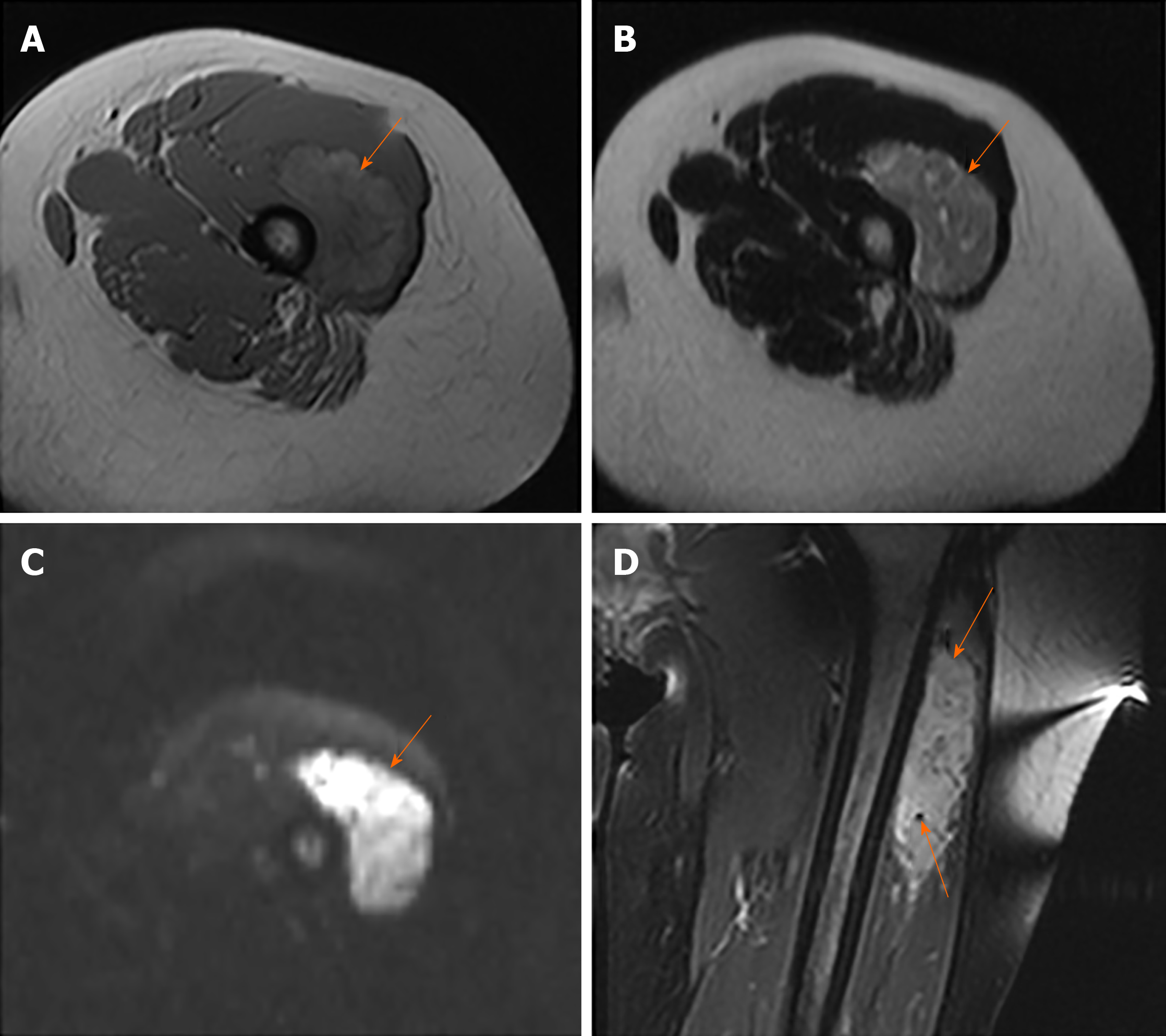
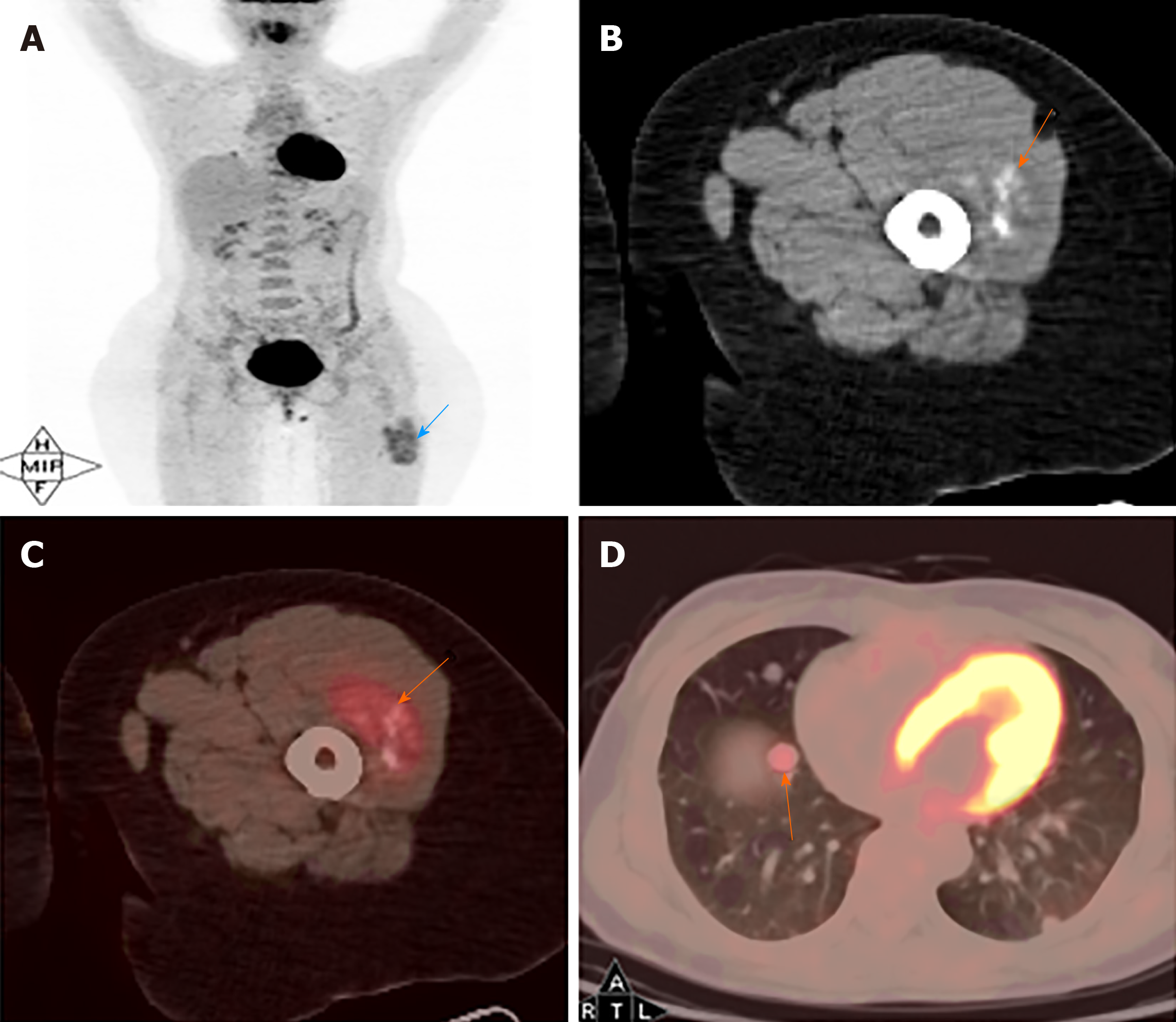
MRI images taken in November 2017 showed a circumscribed mass measuring 5.4 cm × 3.4 cm with hyperintensity on T1- and T2-weighted images and high intensity on DWI images; numerous signal voids (intra-/extra-tumor) were also detected (Figure 1). The initial MRI examination was highly suggestive of hemangioma. Dynamic contrast enhancement of MRI was suggested for further diagnosis, but the patient discharged herself from the hospital against the advice of doctors. The patient was admitted for further treatment due to the enlargement of the mass in June 2018. Subsequent PET/CT examination was performed, and CT showed a slightly hypodense or isodense mass with patchy calcification (Figure 2B). Compared with the initial MRI images, the size of the mass had increased (6.0 cm × 4.1 cm). The PET/CT images showed increased F-deoxyglucose (FDG) uptake with the maximum standardized uptake value of 4.23 for both the mass and several pulmonary nodules, which was highly suggestive of malignant tumors and metastases (Figure 2C and D).
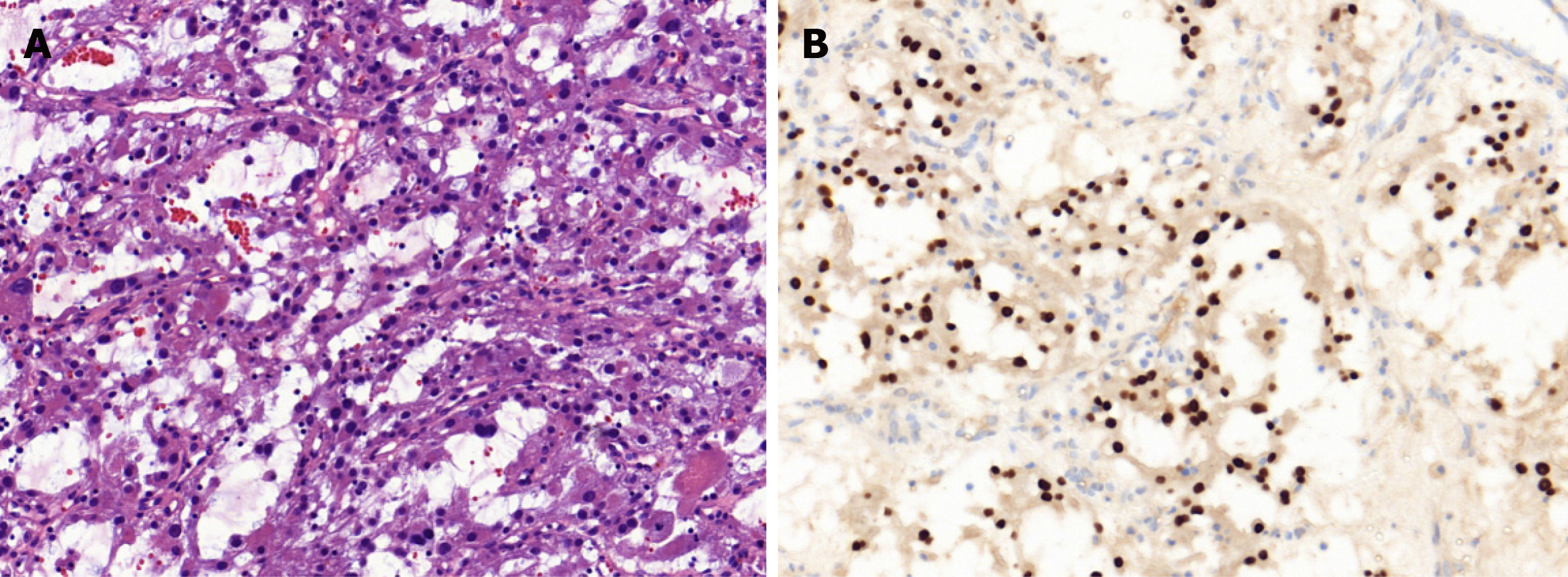
Core needle biopsy was performed 1 wk later. The tumor was diagnosed as ASPS according to the distinct histologic manifestation. The histopathological examination (Figure 3A) showed a pseudoalveolar pattern with nests of large granular cells separated by sinusoidal vascular channels. The immunohistochemical testing (Figure 3B) revealed that the tumor cells were positive for transcription factor binding to immunoglobulin heavy-chain enhancer 3 and negative for S-100 protein, cytokeratin, desmin, and CD56.
The patient had missed the perfect opportunity of surgery and received six cycles of chemotherapy, including ifosfamide, doxorubicin, and Serratia marcescens vaccine for 6 mo.
After chemotherapy, the patient had disease progression. MRI examination in December 2018 revealed an increase in the size (7.6 cm × 5.6 cm) of the tumor and brain metastases. Subsequent pulmonary CT showed multiple nodules and suggested metastases. The patient died 12 mo later.
ASPS is a distinct, malignant soft-tissue tumor characterized by a pseudoalveolar architecture associated with abundant sinusoidal vessels. Adolescents and young adults, aged 15–35 years with a male to female ratio of 1:2, are more likely to be affected[1-3]. ASPS often develops in the lower extremities for adults; however, the head and neck are more frequently involved in children[4-6]. The prognosis of ASPS depends on the presence of metastasis, tumor size, and patient age at the time of diagnosis[7,8]. In the present case, the patient had lung and brain metastasis in the follow-up period and also poor prognosis.
Some studies have demonstrated the imaging characteristics of ASPS. High intensity on T1-weighted images and the presence of numerous signal voids (intra-/extra-tumor) were often emphasized, and these were attributed to the slow-flowing blood and high vascularity[10]. In the present case, the tumor showed similar characteristics on MRI compared with the previous study. The DWI findings of ASPS were rarely reported. ASPS showed the same signal on DWI, as revealed by Cui et al[11]. However, the present case demonstrated high signal intensity, which might be related to abundant cytoplasm and densely arranged cells, leading to the limited diffusion of water molecules.
CT findings are frequently overlooked, and FDG PET/CT findings were rarely reported earlier. No previous study reported calcification; however, some patchy calcification in the mass was detected in the present study, which was easily misdiagnosed as synovial sarcoma. The mechanism of calcification may be associated with calcium deposition caused by histiocytic ischemia and nutritional deficiency. The findings in the present case were consistent with previous findings that ASPS had high FDG uptake[12]. Increased FDG uptake on PET/CT images can help distinguish between benign and malignant tumors[12]. FDG PET/CT makes it possible to distinguish between hemangioma and ASPS more easily. PET/CT examination may also be used for detecting metastasis of the tumor.
ASPS should be distinguished from hemangioma, liposarcoma, and synovial sarcoma. Hemangioma mimicking ASPS may show high signal intensity on T1-weighted images and marked enhancement. However, high FDG uptake on PET/CT image is rarely detected, and DWI usually shows low signal intensity. Although some primary malignant tumors, including liposarcoma and soft-tissue tumors, have high FDG uptake, the characteristic signal intensity on MRI images or density on CT images may be useful in differentiation. Differentiating ASPS with calcification from synovial sarcoma on CT and PET/CT images is difficult, but synovial sarcoma often showed no signal voids on MRI[1,9,13]. Therefore, the combination of these three examinations can provide more clues for the differential diagnosis of ASPS.
In summary, this case highlighted the importance of multimodal imaging examinations in diagnosing ASPS. The possibility of ASPS should be considered when a mass is detected in the soft tissue of the extremities, with hyperintensity and numerous signal voids on T1-weighted, T2-weighted, and DWI images and intense FDG uptake on PET/CT.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan YL S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | Li H, Sun J, Ye J, Wu J. Magnetic resonance imaging and computed tomography features of alveolar soft-part sarcoma in the right deltoid muscle: A case report. Oncol Lett. 2016;11:2857-2860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Christopherson WM, Foote FW, Stewart FW. Alveolar soft-part sarcomas; structurally characteristic tumors of uncertain histogenesis. Cancer. 1952;5:100-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 3. | Martínez R, Niklander S, Deichler J, Leissner O, Seguel H, Esguep A. Alveolar soft-part sarcoma of the masseter and mandibular ramus: Report of a case and review of the literature. J Dent Sci. 2018;13:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Sood S, Baheti AD, Shinagare AB, Jagannathan JP, Hornick JL, Ramaiya NH, Tirumani SH. Imaging features of primary and metastatic alveolar soft part sarcoma: single institute experience in 25 patients. Br J Radiol. 2014;87:20130719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Kim JM, Im SA, Oh SN, Chung NG. Alveolar soft part sarcoma arising from the kidney: imaging and clinical features. Korean J Radiol. 2014;15:381-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Chen J, Chen X, Wang Y, Chen H, Wang Z. Imaging Findings and Histologic Appearances of Alveolar Soft Part Sarcoma in the Prostate: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2015;13:e315-e319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Asano Y, Kashiwagi S, Takada K, Tokimasa S, Takashima T, Ohsawa M, Hirakawa K, Ohira M. Alveolar soft part sarcoma metastatic to the breast: a case report. BMC Surg. 2019;19:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Asokan B, Ramanan P, Sundararaj M, Kovarthini E. Alveolar soft part sarcoma of the forearm. Indian J Plast Surg. 2017;50:310-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Flores RJ, Harrison DJ, Federman NC, Furman WL, Huh WW, Broaddus EG, Okcu MF, Venkatramani R. Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer. 2018;65:e26953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Suh JS, Cho J, Lee SH, Shin KH, Yang WI, Lee JH, Cho JH, Suh KJ, Lee YJ, Ryu KN. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29:680-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Cui JF, Chen HS, Hao DP, Liu JH, Hou F, Xu WJ. Magnetic Resonance Features and Characteristic Vascular Pattern of Alveolar Soft-Part Sarcoma. Oncol Res Treat. 2017;40:580-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Dong A, Wang Y, Cheng C, Zuo C. CT, MRI, and FDG PET/CT in a patient with alveolar soft part sarcoma. Clin Nucl Med. 2014;39:265-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Lin YK, Wu PK, Chen CF, Chen CM, Tsai SW, Chih-Hsueh Chen P, Chen WM. Alveolar soft part sarcoma: Clinical presentation, treatment, and outcome in a series of 13 patients. J Chin Med Assoc. 2018;81:735-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |