Published online Aug 26, 2019. doi: 10.12998/wjcc.v7.i16.2155
Peer-review started: May 21, 2019
First decision: July 30, 2019
Revised: August 6, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: August 26, 2019
Endometriosis (EMs) is a chronic and recurrent, but benign, disease in women of reproductive age, and EMs patients have a high risk of developing gynecological tumors and autoimmune disorders. The etiology of EMs is not clear. Certain genetic markers in the eutopic endometrium are key in the pathogenesis of EMs. MicroRNAs (miRNAs) are implicated in several biological processes, such as cell proliferation, differentiation, and apoptosis. MiR-451 is interesting, as it acts as a tumor suppressor and is relevant to the poor prognosis of cancers.
To evaluate the expression levels and role of miR-451 in the eutopic endometrium and predict possible targets of miR-451 and related signaling pathways.
Quantitative real-time polymerase chain reaction was used to evaluate miR-451 expression in cultured cell lines as well as in pathologic tissues from 40 patients with EMs and 20 donors with no history of the disease (controls). Cell Counting Kit-8 and flow cytometric assays were performed to determine cell proliferation and survival rates after transfection with miR-451 mimics and siRNAs. MiR-451 targets were predicted using miRDB and miRcode target-predicting databases.
We observed lower miR-451 levels in eutopic endometrial tissues from patients with EMs than in control tissues, and this difference was not related to the American Society for Reproductive Medicine stage. Ectopic overexpression of miR-451 in eutopic cells induced apoptosis and inhibited cell proliferation. SiRNA-mediated miR-451 knockdown reversed these effects. Using miRDB and miRcode, we identified 12 potential miR-451 target genes. We hypothesize that the expression of YWHAZ, OSR1, TTN, and CDKN2D may be regulated by miR-451 and be involved in disease pathogenesis.
Reduced miR-451 expression in the eutopic endometrium contributes to the pathogenesis of EMs by promoting cell proliferation and reducing apoptosis. Thus, miR-451 is a novel biomarker for EMs. YWHAZ, OSR1, TTN, and CDKN2D are potential target genes of miR-451 and may have key roles in this disease.
Core tip: Despite the high prevalence of endometriosis (EMs), its etiology is unclear. This study focuses on the expression of miR-451 in patients diagnosed with EMs. We report miR-451 as a novel biomarker of EMs as it is downregulated in the eutopic endometrium. YWHAZ, OSR1, TTN, and CDKN2D were identified as potential target genes of miR-451 that may have important roles in disease pathogenesis. We believe that our study contributes significantly to the literature because it suggests a novel biomarker for EMs that may facilitate the early diagnosis of the disease without the need for invasive methods such as laparoscopic examination.
- Citation: Gao S, Liu S, Gao ZM, Deng P, Wang DB. Reduced microRNA-451 expression in eutopic endometrium contributes to the pathogenesis of endometriosis. World J Clin Cases 2019; 7(16): 2155-2164
- URL: https://www.wjgnet.com/2307-8960/full/v7/i16/2155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i16.2155
Endometriosis (EMs) is a chronic and recurrent, but benign, disease in women of reproductive age, with a morbidity of approximately 10%. It is characterized by the presence of functional endometrial glands and stroma outside the uterine cavity[1,2]. Typical symptoms of EMs include cyclic pelvic pain, dysmenorrhea, dyspareunia, and infertility. Previous studies have reported that EMs patients have a high risk of developing gynecological tumors and autoimmune disorders[3,4]. Thus, EMs can cause severe psychological and physiological harm to those affected by it and imposes a substantial social burden[5,6].
Despite its high prevalence and incapacitating symptoms, the etiology of EMs is not clear. Evidence suggests that it is a multifactorial disease. Retrograde menstruation, immune system disorders, and genetic and environmental factors have been proposed as susceptibility factors for EMs[7-9]. The susceptibility factor of retrograde menstruation proposed by Sampson is the most widely accepted[10]. However, almost all women of reproductive age exhibit some degree of retrograde menstruation, and only 10% to 15% suffer from EMs[11,12]. Recently, more evidence has emerged to support the theory that genetic changes in the eutopic endometrium may be the key molecular events in the pathogenesis of EMs[13].
MicroRNAs (miRNAs) are short noncoding RNA molecules that regulate genetic expression post-transcriptionally and are implicated in several biological processes, such as cell proliferation, differentiation, and apoptosis[14,15]. Some miRNAs have been reported to be abnormally expressed in reproductive cancers[12,16,17], and miR-451 is of particular interest, as it acts as a tumor suppressor and is relevant to the poor prognosis of cancers. Aberrant miR-451 expression has been shown in eutopic and ectopic endometrial tissues; however, data regarding differences in miR-451 expression in the eutopic endometrium from healthy patients and those with EMs remain inconclusive[18,19].
In our study, we examined miR-451 expression in the eutopic endometrium of women with and without EMs and evaluated the role of miR-451 in cell proliferation. Finally, we predicted possible targets of miR-451 and the related signaling pathways.
Pathologic tissues were collected from patients with grade III cervical intraepithelial neoplasia, including 40 with EMs and 20 without. All 60 subjects underwent total hysterectomy at the Shengjing Hospital of China Medical University between 2009 and 2010. The EMs group included 2, 6, 20, and 12 cases at American Society for Reproductive Medicine (ASRM) stage I, II, III, and IV, respectively, of the disease. None of the patients had a history of endocrine, immune, or metabolic disorders and none had received any hormonal or antibiotic treatments within 3 mo prior to surgery.
The ectopic and eutopic endometrial tissues were digested overnight at 37 °C with Dispase IV for 70 min and Dispase II for 50 min (Sigma, United States). After filtration through 100 and 400 mesh nylon screens, the obtained primary cells were rinsed in PBS and then cultured for 24 h in DMEM/F12 medium supplemented with 15% fetal bovine serum and antibiotics at 37 °C in an atmosphere containing 5% CO2.
Total RNA was isolated from the EMs tissues using the TRIzol reagent. cDNA was synthesized from miR-451 using a TaqMan® miRNA Reverse Transcription Kit and used in a 1:5 dilution ratio for qRT-PCR, which was performed, using an miRNA Assays kit and Universal Master Mix following the kit protocols (Applied Biosystems). U6 was used as the endogenous control. Conditions of reverse transcription were as follows: 16 °C for 30 min, 42 °C for 30 min, 85 °C for 5 min, and then holding at 4 °C. Conditions for qRT-PCR were as follows: enzyme activation at 95 °C for 10 min followed by 40 cycles of denaturation for 15 s at 95 °C and annealing and extension for 60 s at 60 °C. All experimental samples were run in triplicate and each qRT-PCR reaction was repeated at least two times. MiR-451 expression levels were calculated and analyzed using the 2−ΔΔCt relative quantitation method.
Using LipofectamineTM 2000 reagent, miR-451 mimics and miR-451 inhibitors were transfected into EMs cells and normal endometrial cells, respectively. The oligonucleotide sequence of the miR-451 mimic is 5′-AAA CCG UUA CCA UUA CUG AGUU-3′, and its NC sequence is 5′-UUC UCC GAA CGU GUC ACG UTT-3′. The oligonucleotide sequence of the miR-451 inhibitor is 5′-AAC UCA GUA AUG GUA ACG GUUU-3′, and the sequence of the scrambled siRNA is 5′-CAG UAC UUU UGU GUA GUA CAA-3’. In addition, cells transfected with or without the empty vector were used as the control groups. All cells were incubated at 37 °C in an atmosphere containing 5% CO2 for 24 to 96 h post transfection.
Cellular proliferation analysis was performed using the Cell Counting Kit-8 (CCK-8) assay. After transfection with miR-451 mimics/inhibitors for 24h, 48h, 72h, and 96 h, 2 × 103 cells were added to 96-well plates and incubated overnight at 37 °C in an atmosphere containing 5% CO2. Then, 10 μL of CCK-8 was added to each well (Beyotime Biotechnology). The cells were incubated for another 4 h at 37 °C in an atmosphere containing 5% CO2, and then cell viability was determined by measuring the optical density at 450 nm.
Annexin V-FITC/PI double-staining assays were performed for analysis of apoptosis. Cells were collected and suspended in PBS 24 h after transfection. Cells were then stained in 500 μL of binding buffer with 5 μL of each of annexin V-FITC and PI (KeyGen Biotech), incubated in the dark at room temperature for 5-15 min, and subjected to flow cytometric analysis to assess cellular apoptosis within 1 h.
Using miRDB (http://mirdb.org/miRDB/index.html) and miRcode (http://www.mircode.org/), we predicted the target genes of miR-451. Expression levels of the identified targeted genes were determined by analyzing the GSE7846 gene profile from the GEO database (https://http://www.ncbi.nlm.nih.gov/geo/). This dataset includes the expression data of endometrial cells derived from patients with EMs (ectopic group) and without EMs (normal group). We screened the differentially expressed genes with a P-value < 0.01 and an adjusted P-value < 0.01 between the EMs and control groups.
Data are expressed as the mean ± SEM. Statistical comparisons between groups were determined using the t-test and χ2 test. Statistical significance was defined as P < 0.05. Analyses were performed using the R 3.4.2 and SPSS 22.0 software.
This study was approved by the China Medical University Research Ethics Committee according to the Helsinki Declaration, and written informed consent was obtained from each study participant.
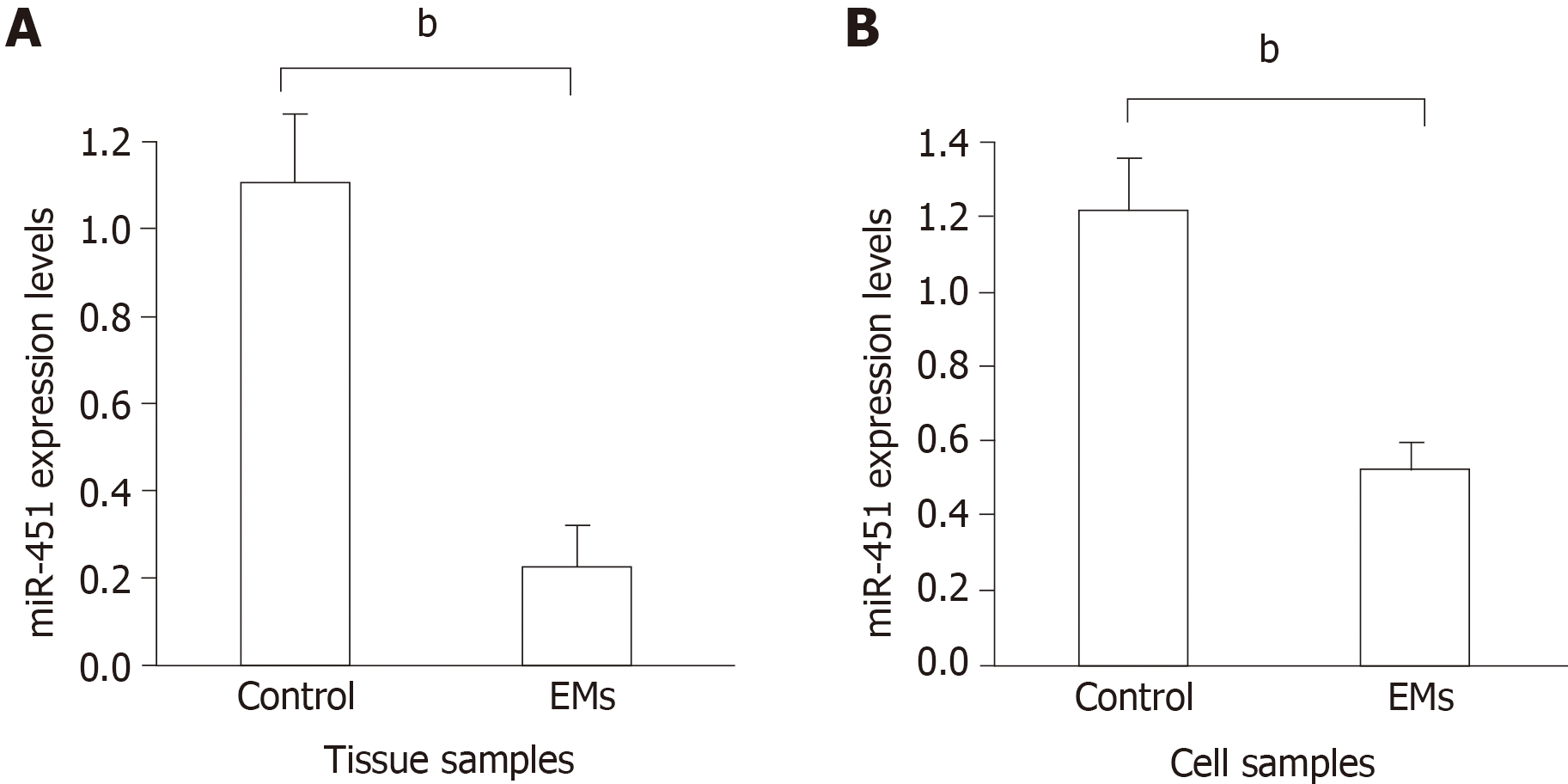
qRT-PCR was performed to quantitatively analyze the expression levels of miR-451 in eutopic tissues from the EMs and control groups. As shown in Figure 1A, we observed a significant reduction in miR-451 expression in the EMs group compared to the control group (EMs, 0.22 ± 0.06; control, 1.12 ± 0.11, P < 0.01). Consistent with the tissue results, miR-451 expression in cells was significantly lower in the EMs group than in the control group (Figure 1B). The correlation of miR-451 expression levels with ASRM stage was then analyzed, as shown in Table 1. No significant association was found between miR-451 expression and ASRM stage (P > 0.05).
| ASRM stage | Cases (n) | MiR-451 level (2-ΔΔCT) | P-value |
| I | 2 | 0.21 | > 0.05 |
| II | 6 | 0.16 | > 0.05 |
| III | 20 | 0.27 | > 0.05 |
| IV | 12 | 0.19 | > 0.05 |
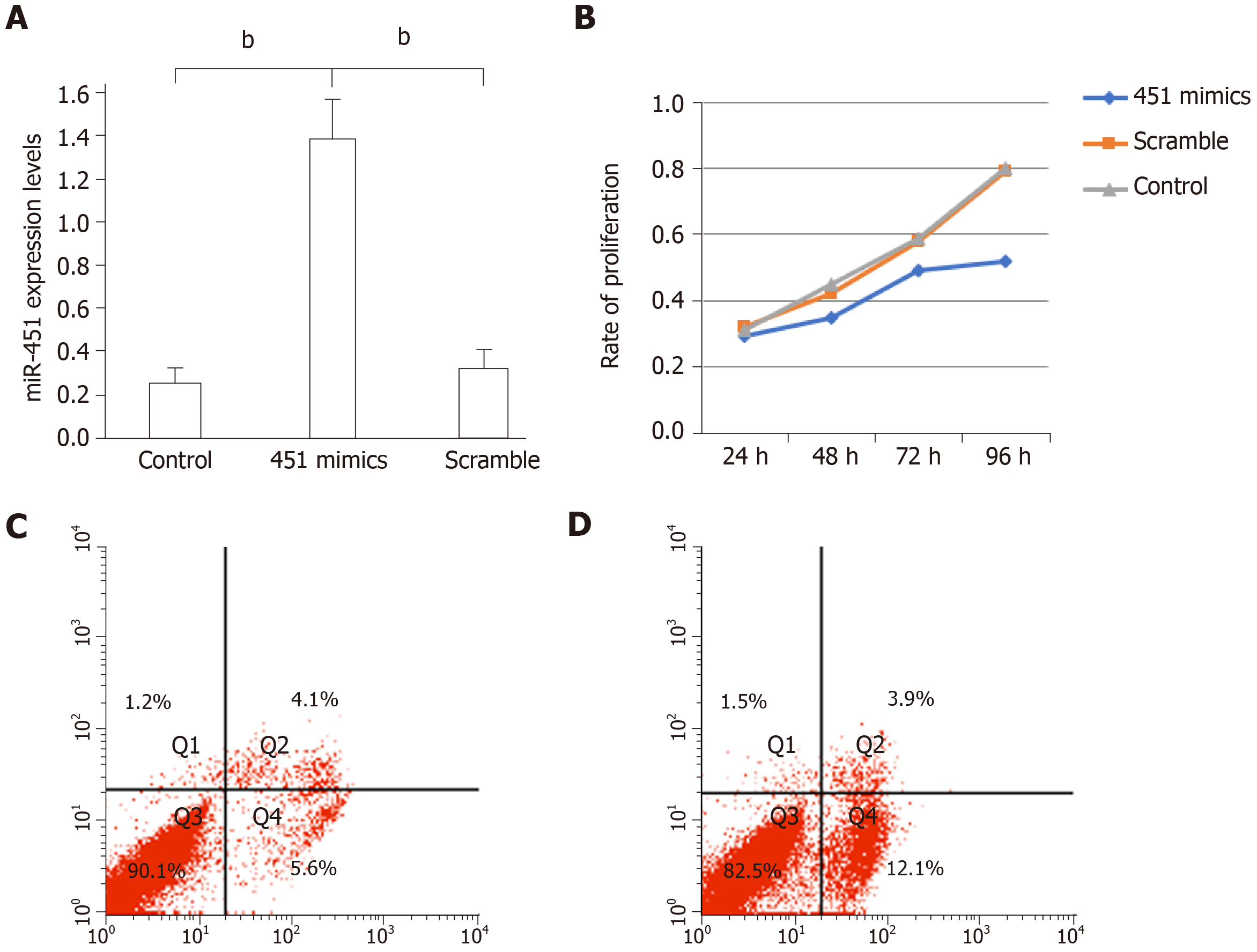
MiR-451 levels in eutopic cells transfected with miR-451 mimic were higher than those in the non-transfected control and scrambled mimic oligomer groups (miR-451 mimic, 1.33 ± 0.28; control, 0.25 ± 0.06; scrambled, 0.32 ± 0.09, P < 0.01) (Figure 2A). CCK-8 assay results showed that transfection with miR-451 mimic suppressed the proliferation rate of EMs cells (Figure 2B). To investigate whether the reduced cell proliferation resulted from apoptosis, we evaluated the effect of miR-451 mimic on cellular apoptosis using flow cytometry. MiR-451 mimic induced early apoptosis in a larger number of cells compared to scrambled oligonucleotides, and this difference was statistically significant (P < 0.01) (Figure 2C and 2D). Thus, overexpression of miR-451 in EMs cells induces apoptosis and inhibits cell proliferation.
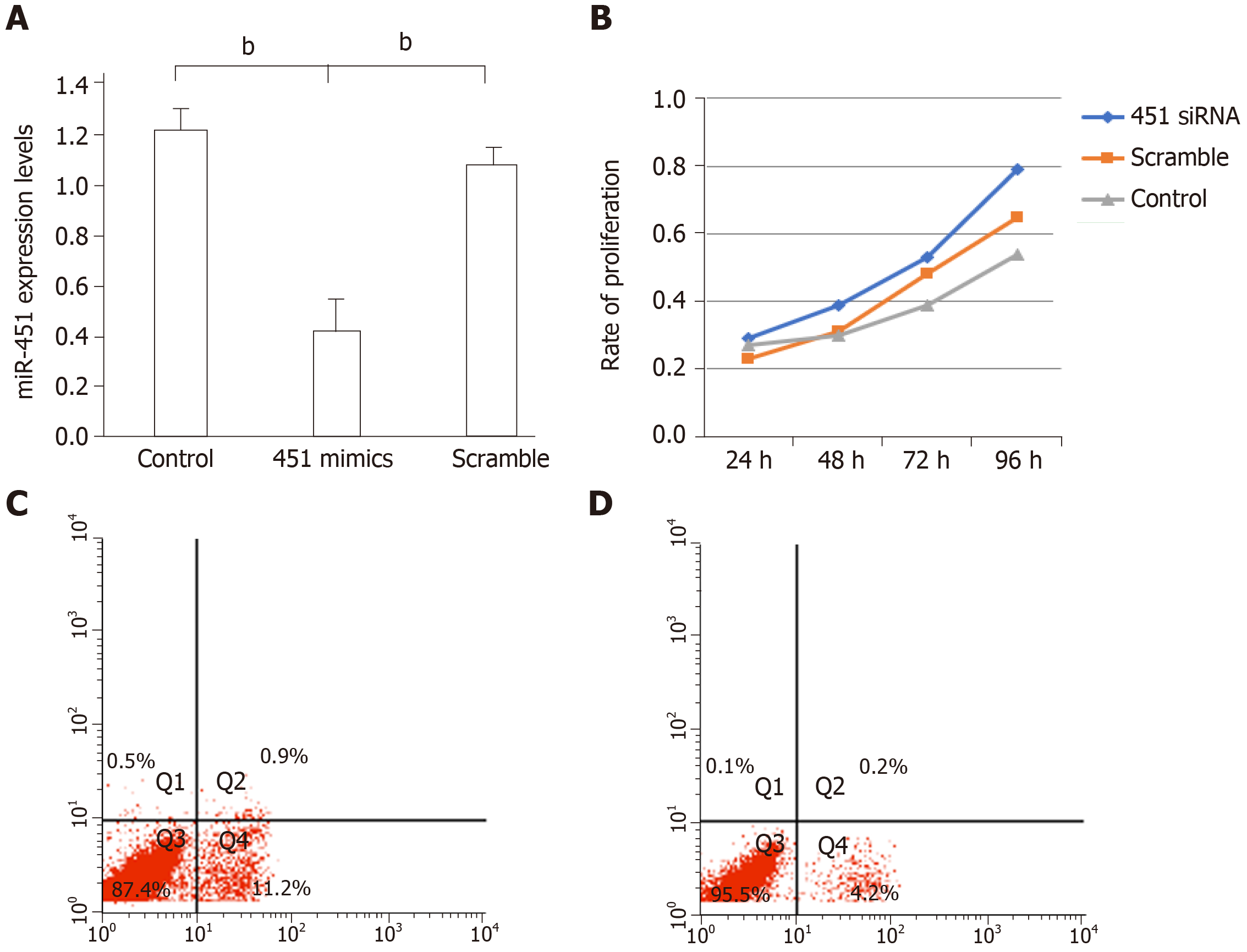
As shown in Figure 3A, miR-451 expression was significantly attenuated in the siRNA-transfected group compared to the non-transfected and scrambled mimic oligomer groups (miR-451 siRNA, 0.41 ± 0.14; control, 1.23 ± 0.08; scrambled, 1.06 ± 0.06, P < 0.01). Additionally, the proliferation ability of miR-451 siRNA-transfected cells was greater than that of the other two groups (Figure 3B). We used flow cytometric assay to evaluate the effect of miR-451 siRNA transfection on apoptosis. Our results showed that the proportion of early apoptotic cells was significantly lower in the miR-451 siRNA group compared to that in the scrambled group (P < 0.01) (Figure 3C and 3D). These results indicate that, in eutopic cells, miR-451 reduces apoptosis and increases cell proliferation.
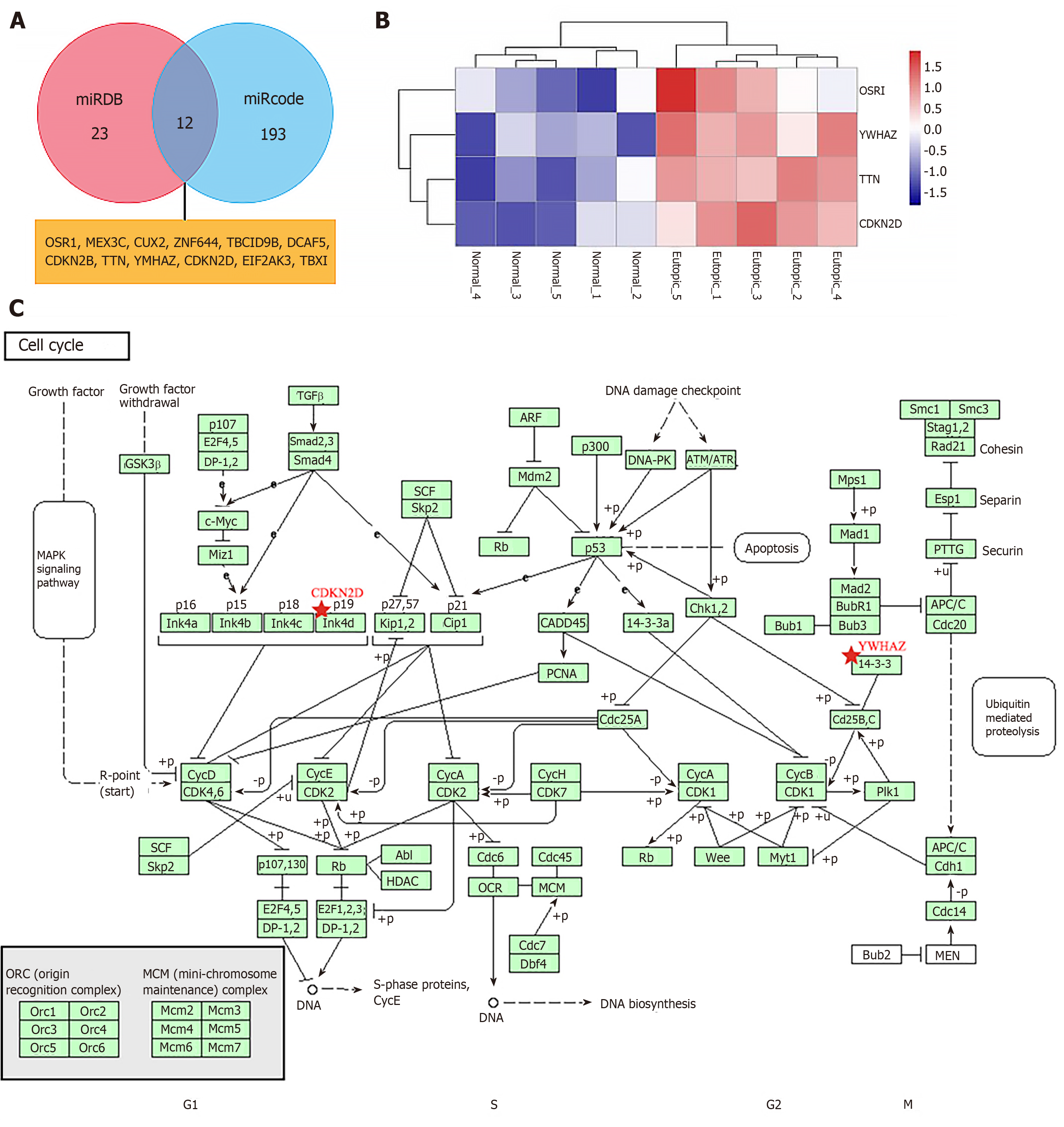
Using the miRDB and miRcode miRNA target prediction databases, we identified a total of 12 genes targeted by miR-451, namely, OSR1, MEX3C, CUX2, ZNF644, TBC1D9B, DCAF5, CDKN2B, TTN, YWHAZ, CDKN2D, EIF2AK3, and TBX1 (Figure 4A). As shown in Figure 4B, among the targeted genes, the expression levels of OSR1, YWHAZ, TTN, and CDKN2D were significantly different between the two groups according to GSE7846 (P < 0.05, adj. P < 0.05). The logFC values of OSR1, YWHAZ, TTN, and CDKN2D were 0.76, 0.43, 0.33, and 0.63, respectively. Furthermore, according to the pathway analysis data in the Kyoto Encyclopedia of Genes and Genomes, YWHAZ and CDKN2D may have important roles in the cell cycle in EMs (Figure 4C).
In this study, qRT-PCR analysis of eutopic endometrial tissues and cells showed that miR-451 was significantly downregulated in patients with EMs compared to normal controls (P = 0.011). Although we did not observe a significant association between miR-451 expression and the ASRM stage of EMs, ectopic overexpression of miR-451 in eutopic cells in EMs was shown to be associated with reduced cell proliferation and increased apoptosis. Conversely, siRNA-mediated knockdown of miR-451 promoted the proliferation and reduced the apoptosis of eutopic cells.
The “eutopic endometrium determinism” theory suggests that the occurrence of EMs is mainly dependent on the characteristics of eutopic endometrial lesions, and retrograde menstruation may act as a precipitating factor. Thus, genetic dysregulation in the endometrium is crucial in the pathogenesis of EMs. Identifying differentially expressed genes between patients with and without EMs would serve as a minimally invasive method to diagnose EMs and evaluate the risk of recurrence. For example, Mahdian et al[20] reported that MIF, CD74, and COX-2 are essential in inflammation and endometrium reconstruction during the menstrual cycle, and increased expression of these genes is a molecular biomarker for the development and pathophysiology of EMs. In addition, Sapkota et al[21] also identified five novel loci (CCDC170, FN1, SYNE1, ESR1, and FSHB) and nineteen independent single nucleotide polymorphisms that are significantly associated with the risk of EMs.
Furthermore, miRNAs regulate the expression of target genes and key cellular processes in EMs. In 2009, Burney et al[14] reported the downregulation of the miR-9 and miR-34 miRNA families in eutopic cells in the setting of EMs, and this downregulation is closely related to progesterone resistance in early secretory endometrium. Laudanski et al[22] showed that miR-483-5p and miR-629-3p are downregulated in EMs, and this is associated with inflammation. Moreover, miR-21 was shown to be significantly upregulated in severe EMs (stage III/IV) compared to mild EMs (stage I/II)[23]. Notably, miR-451 has been established as a tumor-suppressor gene in gastric, colorectal, bladder, and non-small cell lung carcinomas[24], and it was also shown to be downregulated in ovarian cancer compared to its concurrent EMs[25]. In addition, Nothnick et al[26] showed that deficiency of miR-451 regulates fibrinogen alpha chain and reduces endometrial implantation in a mouse model. Similarly, we found that miR-451 was downregulated in the eutopic endometrium in EMs compared to normal controls in studies involving both tissues and cells.
Using miRNA target-predicting databases, we identified 12 potential target genes of miR-451 and analyzed their expression levels according to the GSE7846 dataset. Finally, a total of four genes, YWHAZ, OSR1, TTN, and CDKN2D, were selected for further analysis. Among these target genes of miR-451, YWHAZ has previously been shown to be overexpressed in tissues in EMs[19,27]. Joshi et al[19] reported that miR-451 regulates YWHAZ expression and promotes proliferation of eutopic cells in baboons with EMs[19]. However, the roles of OSR1, TTN, and CDKN2D in EMs have not been reported until now. Published reports suggest that OSR1 inhibits proliferation and induces cellular apoptosis by acting on the WNK and NF-κB pathways, and OSR1 is dramatically downregulated in several carcinomas[28-30]. In addition, CDKN2D has been shown to be involved in carcinogenesis and has been identified in gynecological cancers. This gene may be regulated by miR-451 in esophageal carcinoma cell lines[31]. Thus, our study provides several novel therapeutic targets for EMs.
Notably, most studies on EMs have only focused on identifying differences between ectopic lesions and eutopic endometrium. For example, Graham et al[18] reported that miR-451 is overexpressed in ectopic lesions compared to eutopic lesions and reduces cell survival by regulating MIF. In this study, we found significant differences in miR-451 expression in the eutopic endometrium of patients with and without EMs, which effectively supports the “eutopic endometrium determinism” theory. Furthermore, we identified four potential target genes of miR-451 by bioinformatics analysis and analyzed their downstream pathways.
Our study has two limitations. First, the number of included patients was relatively small. Second, the in silico-predicted targets of miR-451 need to be validated through experiments, such as 3′-UTR luciferase reporter assays. However, we believe that our results indicate a novel role of miR-451 in EMs and support several potential biomarkers in the form of miR-451 targets that may be used for future clinical diagnosis and therapy of this disease.
In conclusion, miR-451 is a novel biomarker for EMs and is downregulated in the eutopic endometrium. YWHAZ, OSR1, TTN, and CDKN2D are potential target genes of miR-451 and may have important roles in the pathogenesis of EMs.
Despite the high prevalence of endometriosis (EMs), its etiology is unclear.
MiR-451 acts as a tumor suppressor and is relevant to the poor prognosis of cancers.
To evaluate the expression levels and role of miR-451 in the eutopic endometrium and predict possible targets of miR-451 and related signaling pathways.
Quantitative real-time PCR was used to evaluate miR-451 expression. Cell Counting Kit-8 and flow cytometric assays were performed to determine cell proliferation and survival rates.
MiR-451 was downregulated in the eutopic endometrium and related with EMs cell proliferation and apoptosis. YWHAZ, OSR1, TTN, and CDKN2D were identified as potential target genes of miR-451.
Reduced miR-451 expression in the eutopic endometrium contributes to the pathogenesis of EMs by promoting cell proliferation and reducing apoptosis.
MiR-451 is a novel biomarker for EMs. YWHAZ, OSR1, TTN, and CDKN2D are potential target genes of miR-451 and may have key roles in this disease.
Conflict of interest statement: All authors declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jorge AG, Orbell JH S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 609] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 3. | Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Somigliana E, Vigano' P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Gao X, Yeh YC, Outley J, Simon J, Botteman M, Spalding J. Health-related quality of life burden of women with endometriosis: a literature review. Curr Med Res Opin. 2006;22:1787-1797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 587] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 7. | Pitt JA, Feng L, Abbott BD, Schmid J, Batt RE, Costich TG, Koury ST, Bofinger DP. Expression of AhR and ARNT mRNA in cultured human endometrial explants exposed to TCDD. Toxicol Sci. 2001;62:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Nowak NM, Fischer OM, Gust TC, Fuhrmann U, Habenicht UF, Schmidt A. Intraperitoneal inflammation decreases endometriosis in a mouse model. Hum Reprod. 2008;23:2466-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Avcioğlu SN, Altinkaya SÖ, Küçük M, Demircan-Sezer S, Yüksel H. Can platelet indices be new biomarkers for severe endometriosis? ISRN Obstet Gynecol. 2014;2014:713542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93-110.43. [PubMed] [Cited in This Article: ] |
| 11. | Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010;2010:369549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Braza-Boïls A, Marí-Alexandre J, Gilabert J, Sánchez-Izquierdo D, España F, Estellés A, Gilabert-Estellés J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Majid S, Saini S, Dar AA, Hirata H, Shahryari V, Tanaka Y, Yamamura S, Ueno K, Zaman MS, Singh K, Chang I, Deng G, Dahiya R. MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal cancer. Cancer Res. 2011;71:2611-2621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Marí-Alexandre J, García-Oms J, Barceló-Molina M, Gilabert-Aguilar J, Estellés A, Braza-Boíls A, Gilabert-Estellés J. MicroRNAs and angiogenesis in endometriosis. Thromb Res. 2015;135 Suppl 1:S38-S40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Cohn DE, Fabbri M, Valeri N, Alder H, Ivanov I, Liu CG, Croce CM, Resnick KE. Comprehensive miRNA profiling of surgically staged endometrial cancer. Am J Obstet Gynecol. 2010;202:656.e1-656.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Bianchi N, Zuccato C, Finotti A, Lampronti I, Borgatti M, Gambari R. Involvement of miRNA in erythroid differentiation. Epigenomics. 2012;4:51-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Graham A, Falcone T, Nothnick WB. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum Reprod. 2015;30:642-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, Lessey BA, Fazleabas AT. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod. 2015;30:2881-2891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Mahdian S, Aflatoonian R, Yazdi RS, Yaghmaei P, Ramazanali F, Afsharian P, Shahhoseini M. Macrophage migration inhibitory factor as a potential biomarker of endometriosis. Fertil Steril. 2015;103:153-9.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, O D, Peterse D, Rexrode KM, Ridker PM, Schork AJ, MacGregor S, Martin NG, Becker CM, Adachi S, Yoshihara K, Enomoto T, Takahashi A, Kamatani Y, Matsuda K, Kubo M, Thorleifsson G, Geirsson RT, Thorsteinsdottir U, Wallace LM; iPSYCH-SSI-Broad Group, Yang J, Velez Edwards DR, Nyegaard M, Low SK, Zondervan KT, Missmer SA, D'Hooghe T, Montgomery GW, Chasman DI, Stefansson K, Tung JY, Nyholt DR. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 2017;8:15539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 22. | Laudanski P, Charkiewicz R, Kuzmicki M, Szamatowicz J, Charkiewicz A, Niklinski J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reprod Biol Endocrinol. 2013;11:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2011;18:229-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12:1153-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Wu RL, Ali S, Bandyopadhyay S, Alosh B, Hayek K, Daaboul MF, Winer I, Sarkar FH, Ali-Fehmi R. Comparative Analysis of Differentially Expressed miRNAs and their Downstream mRNAs in Ovarian Cancer and its Associated Endometriosis. J Cancer Sci Ther. 2015;7:258-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Nothnick WB, Graham A, Holbert J, Weiss MJ. miR-451 deficiency is associated with altered endometrial fibrinogen alpha chain expression and reduced endometriotic implant establishment in an experimental mouse model. PLoS One. 2014;9:e100336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Vestergaard AL, Knudsen UB, Munk T, Rosbach H, Martensen PM. Transcriptional expression of type-I interferon response genes and stability of housekeeping genes in the human endometrium and endometriosis. Mol Hum Reprod. 2011;17:243-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Otani K, Dong Y, Li X, Lu J, Zhang N, Xu L, Go MY, Ng EK, Arakawa T, Chan FK, Sung JJ, Yu J. Odd-skipped related 1 is a novel tumour suppressor gene and a potential prognostic biomarker in gastric cancer. J Pathol. 2014;234:302-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Yuan Y, Liang P, Guo X, Ying Y, Shu XS, Gao M, Cheng Y. OSR1 is a novel epigenetic silenced tumor suppressor regulating invasion and proliferation in renal cell carcinoma. Oncotarget. 2017;8:30008-30018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Chen W, Wu K, Zhang H, Fu X, Yao F, Yang A. Odd-skipped related transcription factor 1 (OSR1) suppresses tongue squamous cell carcinoma migration and invasion through inhibiting NF-κB pathway. Eur J Pharmacol. 2018;839:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Zang WQ, Yang X, Wang T, Wang YY, Du YW, Chen XN, Li M, Zhao GQ. MiR-451 inhibits proliferation of esophageal carcinoma cell line EC9706 by targeting CDKN2D and MAP3K1. World J Gastroenterol. 2015;21:5867-5876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |