Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11101
Peer-review started: May 31, 2022
First decision: July 12, 2022
Revised: July 22, 2022
Accepted: September 12, 2022
Article in press: September 12, 2022
Published online: October 26, 2022
Klebsiella pneumoniae (K. pneumoniae) is a clinically common Gram-negative bacillus that can cause community- and hospital-acquired infections and lead to pneumonia, liver abscesses, bloodstream infections, and other infectious diseases; however, severe pneumonia caused by hypervirulent K. pneumoniae (hvKp) complicated by acute intra-abdominal multiple arterial thrombosis and bacterial embolism is rarely seen in the clinical setting and has not been reported in the literature.
A 51-year-old man was hospitalized with fever and dyspnea. Persistent mild pain in the middle and upper abdomen began at dawn on the 3rd day following admission and developed into persistent severe pain in the left upper abdomen 8 h later. Based on chest computed tomography (CT), bronchoscopy, bronchoalveolar lavage fluid metagenomic next-generation sequencing, abdominal aortic CT angiography (CTA), and culture of the superior mesenteric artery embolus, adult community-acquired severe hvKp pneumonia complicated by acute intra-abdominal multiple arterial thrombosis and bacterial embolism was diagnosed. Notably, he recovered and was discharged from the hospital after receiving effective meropenem anti-infection, endovascular contact thrombolytic, and systemic anticoagulant therapies and undergoing percutaneous thrombus aspiration. Ten days later, the patient returned to the hospital for abdominal CTA examination, which indicated blocked initial common pathway of the celiac trunk and superior mesenteric artery, and local stenosis. Therefore, celiac trunk artery stenting was performed in Chongqing Hospital, and postoperative recovery was good.
We report a case of hvKp severe pneumonia complicated by acute intra-abdominal multiple arterial thrombosis and bacterial embolism and suggest that clinicians should consider the possibility of a Gram-negative bacillus infection and conduct effective pathogen detection in a timely fashion when managing patients with severe community-acquired pneumonia before obtaining bacteriologic and drug sensitivity results. At the same time, when patients have severe pulmonary infection complicated by severe abdominal pain, an acute mesenteric artery embolism should be considered to avoid delays in treatment.
Core Tip: The community-acquired Klebsiella pneumoniae (K. pneumoniae) infection rate is relatively low, but a hypervirulent K. pneumoniae infection can develop into a severe illness or even death, which deserves the attention of clinicians. At the same time, although current thrombolytic therapy for the arterial system complicated by infectious bacterial embolism is controversial, we believe that local thrombolytic therapy is still effective under effective anti-infection therapy and warrants further study.
- Citation: Bao XL, Tang N, Wang YZ. Severe Klebsiella pneumoniae pneumonia complicated by acute intra-abdominal multiple arterial thrombosis and bacterial embolism: A case report. World J Clin Cases 2022; 10(30): 11101-11110
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11101.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11101
Hypervirulent Klebsiella pneumoniae (hvKp) are highly virulent variants of K. pneumoniae, and these strains can cause purulent liver abscesses and bacteremia in the absence of biliary or intestinal pathology, as well as spread to the lungs, eyes, and central nervous system, and cause severe community-acquired infections in previously healthy and young people, such as liver abscesses, pneumonia, meningitis, endophthalmitis, and necrotizing fasciitis[1-3].
Acute mesenteric ischemia (AMI) is one of the most severe acute abdominal diseases encountered in vascular surgery. AMI is caused by obstruction of the mesenteric artery or vein, which leads to a sudden interruption of the blood supply or reflux, obstruction of the blood supply and malnutrition of the intestinal canal, and finally the loss of bowel function and necrosis. AMI has an insidious onset and rapid progression and causes severe consequences. If diagnosis and treatment cannot be made in a timely fashion, the fatality rate can reach 50%-70%[4].
A 51-year-old nonsmoking male chemical plant worker with normal immune function was hospitalized on March 28, 2021 with fever and dyspnea for 4 d.
Four days before admission, the patient developed a fever without any apparent trigger. His peak temperature was 39.3 °C, and he experienced progressive aggravation of dyspnea and fatigue. In addition, the patient had a paroxysmal, nonproductive cough.
Hypertension existed for 10 d. Amlodipine besylate tablets (5 mg/d) were taken orally for antihypertensive treatment daily.
His personal and family histories were unremarkable.
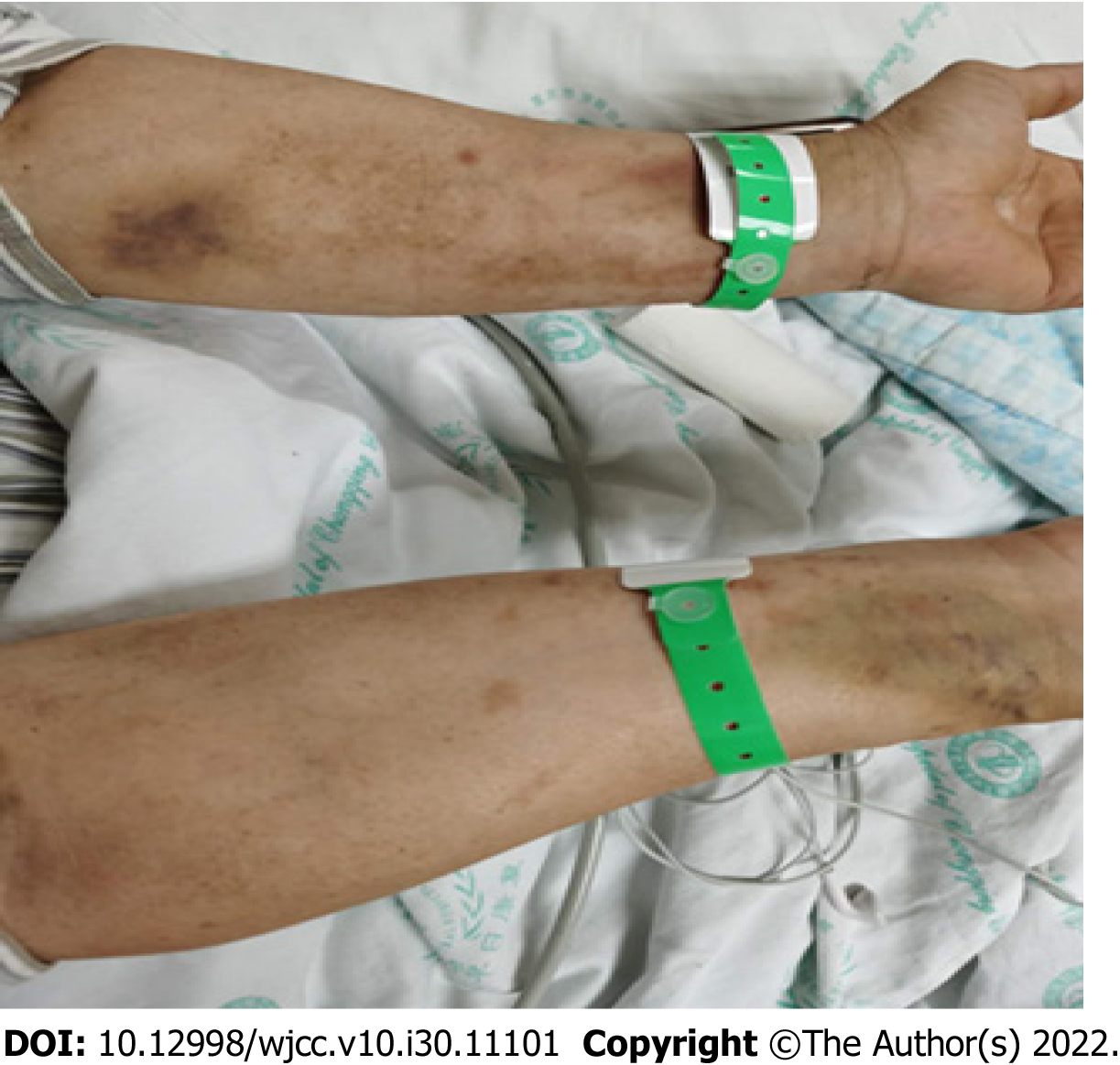
The initial physical examination showed a heart rate of 107 beats/min, a respiratory rate of 33 breaths/min, a body temperature of 39.1 °C, and a blood pressure of 20.62/10.0 kPa. The body weight was 65 kg. Scratches were noted on both the forearms and hands, and skin ulcerations were occasionally observed (Figure 1). Coarse breath sounds and extensive rales were appreciated in both lungs. The remainder of the organ systems were unremarkable on physical examination.
Laboratory testing revealed the following: WBC count, 20.30 × 109/L; neutrophil percentage, 84.4%; hemoglobin concentration, 116 g/L; procalcitonin, 2.03 ng/mL; C-reactive protein (CRP), 380.00 mg/L; interleukin-6 (IL-6), 408.4 pg/mL; serum albumin, 25.0 g/L; D-dimer, 1.84 mg/L; arterial blood gas (FiO2, 33%; pH, 7.46; PaCO2, 35 mmHg; PaO2, 76 mmHg; HCO3, 24.9 mmol/L; lactic acid, 1.1 mmol/L; and P/F, 230 mmHg. The following measures and indicators were normal: liver function; coagulation function; HIV; HBV; A/B virus antigen; cytomegalovirus IgM/IgG; Epstein-Barr virus IgM/IgG; blood lipids; blood glucose; lymphocyte subsets; IgM, IgG, IgA, IgE, IgD; renal function; autoantibody spectrum; anti-neutrophil cytoplasmic antibody spectrum; brain natriuretic peptide; myocardial injury markers; ECG; and routine defecation. In addition, there were no abnormalities detected in the heart, abdominal and lower limb deep veins by ultrasonography, or by bilateral carotid artery ultrasonography.
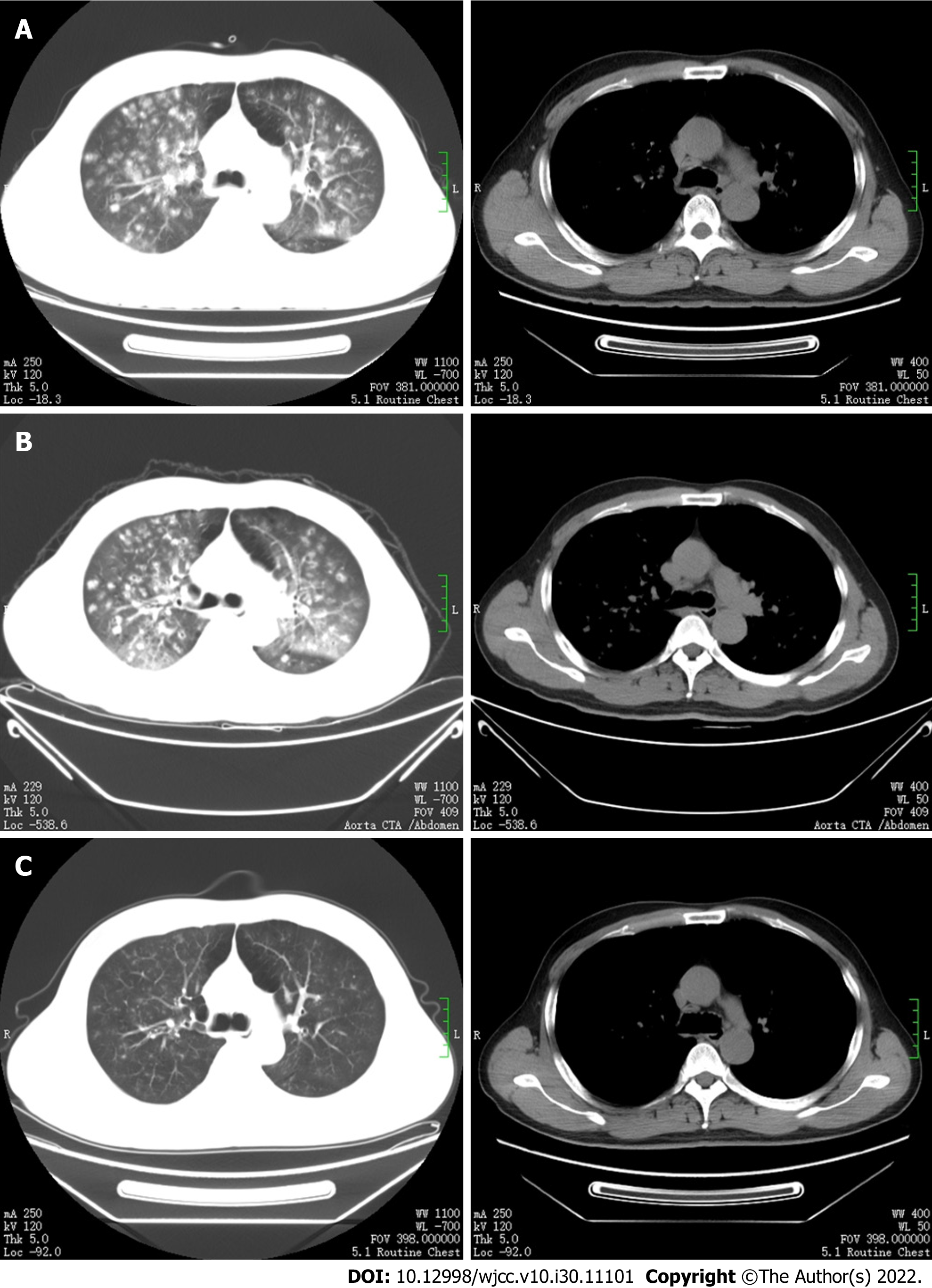
On the day of admission, chest computed tomography (CT) showed multiple patchy, nodular, flocculent high-density shadows in both lungs, with blurred edges and small voids in some lesions (Figure 2A).
We made an initial diagnosis of adult community-acquired severe pneumonia and type I respiratory failure; however, the pathogenesis was not determined.
After admission, the patient was administered high-flow nasal cannula supportive therapy, piperacillin/tazobactam (4.5 g intravenous infusion every 8 h), and linezolid (0.6 g intravenous infusion every 12 h).
The patient had recurrent high fevers, and the P/F decreased to 152 mmHg. During this period, sputum and blood cultures were repeatedly performed, but no causative organisms were isolated. In addition, in the early morning of the 3rd day following admission, the patient began to develop persistent mild pain in the middle and upper abdomen, although no positive signs were elicited on the abdominal physical examination. The pain was not relieved by intravenous proton pump inhibitor therapy. Eight hours later, the patient developed persistent severe pain in the left upper abdomen; abdominal signs on physical examination were still negative.
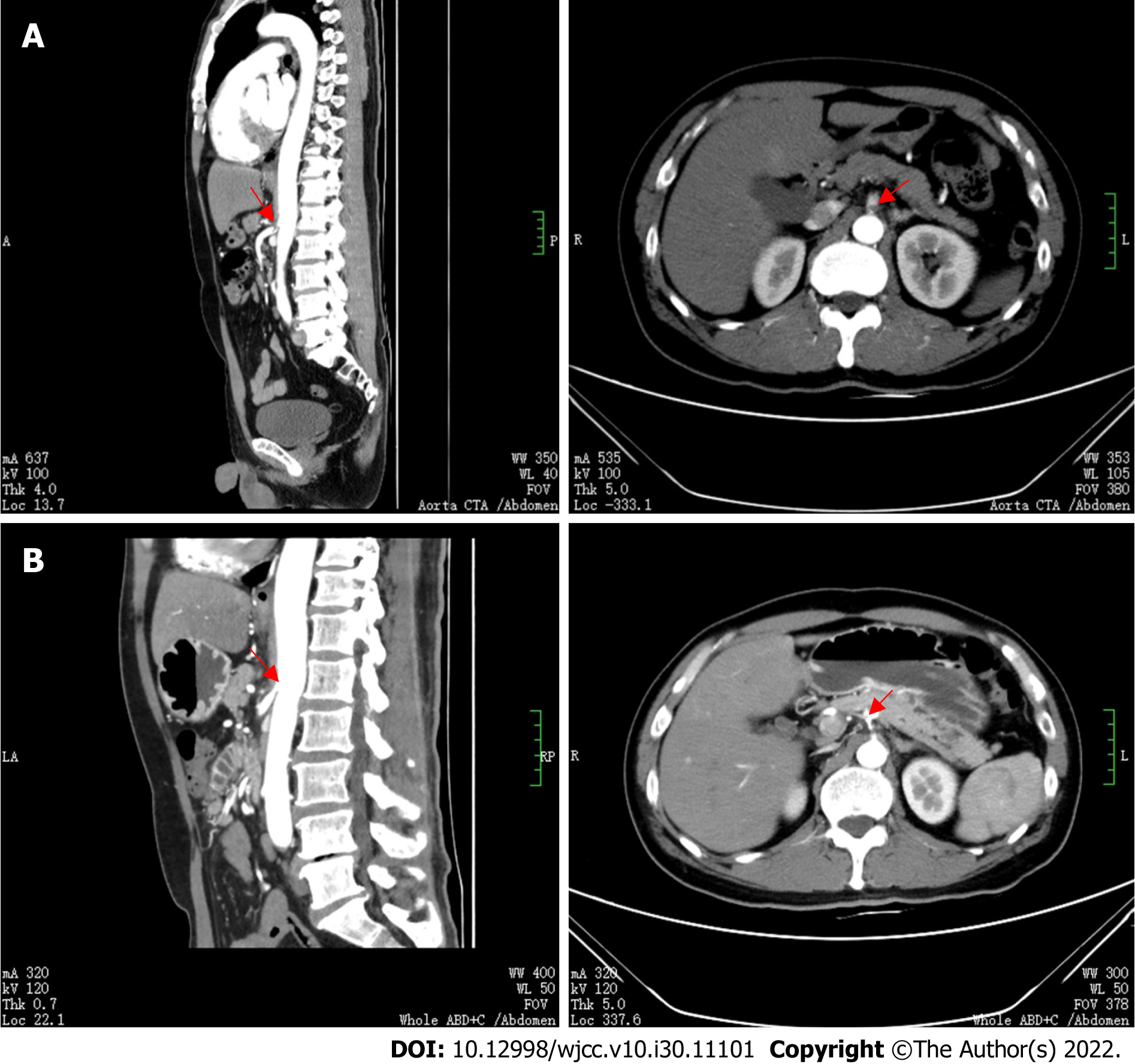
An emergency determination of myocardial injury markers, serum amylase, serum lipase, and re-examination of the cardiac and whole abdominal ultrasounds were normal, but the plasma D-dimer increased to 25.5 mg/L. The results of repeat chest and abdominal CT angiography (CTA) showed that the proximal segment of the celiac trunk and superior mesenteric artery were embolized, and the distal branch appeared reduced in size. The hepatic parenchyma around the gallbladder was enhanced in the arterial stage, with uneven local perfusion and a few calcified plaques in the abdominal aorta (Figure 3A). The bilateral lung lesions increased, and the solid parts around some nodules increased, with reverse halo signs and trophovascular signs (Figure 2B).
The patient had no signs of intestinal necrosis and peritonitis and no indications for a laparotomy; however, to avoid bowel resection, multiple intra-abdominal artery emboli require urgent restoration of the blood supply.
The acute intra-abdominal multiple arterial emboli were clear, and endovascular catheter treatment was recommended; however, the patient had severe pneumonia, and it is not clear what type the embolus was. Indeed, if the embolus is bacterial, thrombolytic therapy may cause systemic spread of bacteria, thus aggravating the patient’s overall status.
The patient was in an early stage of acute abdominal multiple artery emboli without obvious peritoneal irritation, and the lesions were located in the main trunk. Therefore, intracavity catheter therapy combined with systemic anticoagulation could be a first treatment choice; however, the patient had severe pneumonia, and if the intra-abdominal artery was bacterially embolized, thrombolytics increased the risk of systemic bacterial spread, resulting in further aggravation of the infection or life-threatening complications.
The patient had severe pneumonia with acute intra-abdominal multiple arterial emboli; thus, his current condition was severe. Although the etiology was unknown, it is not possible to know whether there were bacterial emboli. If the blood supply was not restored in a timely fashion, irreversible loss of the intestinal canal would occur, further aggravating the patient’s condition. Therefore, endovascular catheter treatment and local intracatheter arterial thrombolysis were recommended on the basis of the patient undergoing strong anti-infection therapy. During the operation, aspirated emboli could be sent for culture. In addition, bronchoalveolar lavage fluid metagenomic next-generation sequencing (BALF-mNGS) was performed to further identify the pathogen(s) after the patient’s general condition improved.
We made a final diagnosis of community-acquired severe pneumonia with acute intra-abdominal multiple arterial emboli.
We replaced the antibiotics with meropenem (1 g intravenously every 8 h) combined with vancomycin (1 g intravenously every 12 h) for anti-infection, initiated low-molecular weight heparin sodium (100 IU/kg subcutaneously for anticoagulation every 12 h), withheld food, and provided fluid rehydration and parenteral nutrition. We immediately performed artery digital subtraction angiography (DSA) and found that the embolus in the superior mesenteric artery opening had almost completely blocked the blood vessels.
We implanted a percutaneous endovascular catheter to aspirate the superior mesenteric artery emboli. The aspirated emboli were sent for microbial culture, and endovascular contact thrombolysis [urokinase (300000 U)] was performed. After surgery, urokinase was pumped with 100000 U arterial microbeam for 4 h, followed by a conventional heparin (1250 U) arterial micropump for 4 h by alternate intra-arterial catheter administration, and systemic low-molecular weight heparin was injected subcutaneously for anticoagulation.
Postoperative abdominal pain was significantly relieved, but abdominal distention appeared on the first day after surgery; thus, the alternate intra-arterial catheter administration of intra-arterial thrombolysis and anticoagulant was continued as before. After 72 h of thrombolytic therapy, intraarterial thrombolytic therapy and intracatheter anticoagulation were discontinued due to positive fecal occult blood, but subcutaneous injection of 100 IU/kg low-molecular-weight heparin every 12 h was continued.
On the 5th d following admission, the superior mesenteric artery embolus culture suggested K. pneumoniae growth (PHOENIX100 Fully Automatic Microbial Analysis System, from BioMérieux, France). On the 7th d following admission, the patient still had intermittent fevers. Therefore, BALF-mNGS (Kingmed Diagnostics, China) was performed to further clarify the etiology, and the results showed that the sequence number of Klebsiella was 13636, among which the sequence number of K. pneumoniae detected was 4812. Therefore, we discontinued vancomycin and continued meropenem to fight the infection, and low-molecular-weight heparin 100 IU/kg subcutaneous injection was continued every 12 h during hospitalization.
The dyspnea was relieved, the body temperature returned to normal, the abdominal pain resolved, abdominal distension improved, and a semifluid diet was resumed. On the 23rd d following admission, a chest CT re-examination showed that the bilateral lung lesions were significantly absorbed and reduced compared with the former CT findings (Figure 2C). The patient's condition improved, and he was discharged home. He was treated with rivaroxaban (15 mg twice daily) and clopidogrel (75 mg once daily). Although the abdominal distention improved, he still had symptoms of abdominal distension after discharge.
Ten days later, the patient returned to the hospital for abdominal CTA examination, which indicated reperfusion of the proper hepatic artery, partial infarction of the spleen, cystic changes, blocked initial common pathway of the celiac trunk and superior mesenteric artery, and local stenosis (Figure 3B). Therefore, celiac trunk artery stenting was performed in Chongqing Hospital, and postoperative recovery was good.
In recent years, the K. pneumoniae detection rate has been increasing steadily. K. pneumoniae belongs to Klebsiella Enterobacteriaceae. According to its virulence and pathogenic characteristics, K. pneumoniae can be divided into classic K. pneumoniae (cKp) and hvKp[5]. One of its main characteristics is that cKp causes nosocomial infection and usually infects immunocompromised or immunocompromised people since cKp carry fewer virulence genes and are less virulent but can show high levels of multiresistance to antibiotics. However, hvKp often manifests as a community-acquired infection, which is more likely to cause disease in healthy people and is often accompanied by multisite invasive infection, which can be life-threatening in severe cases[6]. Compared with cKp strains, hvKp strains showed stronger virulence in various infection modes. Recent studies suggested the existence of hvKp strains with a negative string test. The K1 and K2 capsular serotypes rmpA, rmpA2, aerobactin and yersiniabactin are the common factors responsible for hypervirulent phenotypes[7].
hvKp was initially found mainly in Southeast Asia, but an increasing number of cases have been reported around the world, including in Europe and the United States, and China is a high-incidence area of hvKp infection[8]. A bloodstream source is currently the most recognized route of infection for hvKp. Due to the ability of hvKp to resist neutrophil phagocytosis, it can flow to various tissues and organs in the body through the blood circulation, thereby leading to infection[9].
Chest CT in patients with community-acquired K. pneumoniae pneumonia may show large, honeycomb, patchy consolidation shadows or be accompanied by abscesses, cavities, and liquefaction necrosis, and > 50% of patients have pleural effusions[10]. Pneumonia caused by hematogenous disseminated K. pneumoniae infections is similar to Staphylococcus aureus (S. aureus) infection, such as multiple nodules near the pleura of both lungs, or accompanied by hollow nodules, trophovascular signs, anti-halo signs, and other CT imaging features[11]. Therefore, there is a risk of failure of antibiotic therapy based only on the characteristics of chest CT images.
The patient presented herein was a healthy Asian man who worked as a cleaner in a factory. Before the disease onset, his skin was exposed to sewage pollutants due to touching it with both hands during cleaning, and the skin of his forearms and hands was scratched, resulting in local suppurative infections, after which he developed fevers and dyspnea. His procalcitonin, CRP, and IL-6 Levels were elevated. Chest CT showed multiple patchy, nodular, and flocculent bilateral lung high-density shadows, with blurred edges, and small cavities in some lesions. During chest and abdominal CTA, the lung lesions showed reverse halo and trophovascular signs.
An S. aureus infection was suspected as the initial clinical diagnosis, and treatment with piperacillin/tazobactam combined with linezolid failed. In recent years, mNGS has been shown to be able to rapidly and objectively detect various pathogenic microorganisms in clinical samples and conduct drug resistance gene detection of bacteria; thus, mNGS is widely used in clinical practice[12]. In our case, when early anti-infection treatment was ineffective, BALF was obtained for mNGS detection, and K. pneumoniae was detected, which was consistent with the results of the DSA aspiration embolus culture, proving that meropenem treatment was effective and reducing the adverse effects caused by the blind use of other broad-spectrum antibiotics.
Although the traditional research view is that high virulence and multidrug resistance do not overlap in the hvKp genome, most hvKp remain highly sensitive to commonly used antibiotics except ampicillin, and their resistance rate is extremely low[7,13]. However, with the extensive use of antibiotics, drug-resistant hvKp has emerged, especially carbapenem-resistant hvKp (CR-hvKp), which has brought great challenges to clinical treatment. Although our case was not detected as CR-hvKp, the application of meropenem was effective, but in the future, while treating hvKp invasion syndrome in a timely and standardized manner, we should also strengthen CR-hvKp detection and nosocomial infection prevention and control measures to prevent the spread of CR-hvKp infection[14,15].
AMI has an insidious onset and rapid progression. Various causes lead to acute obstruction of the mesenteric artery or vein, which leads to sudden interruption of the blood supply or reflux, intestinal blood supply disorder and malnutrition, and eventually loss of function and necrosis[4]. Although the clinical incidence is only 1%-2%, the fatality rate is very high[16,17]. Sixty-five percent of AMI patients have acute superior mesenteric artery emboli[18]. Mesenteric artery emboli mostly originate from the left atrium and are often associated with arrhythmias, such as atrial fibrillation, and can also be caused by heart valve dysfunction or bacterial emboli shedding. It has been reported that high-risk factors include atherosclerosis, dyslipidemia, hypertension, dehydration, diabetes, estrogen, and antiphospholipid syndrome[19].
Although the chief complaint of severe abdominal pain inconsistent with the physical examination findings is a classic presentation of early AMI, it is not sufficient for its diagnosis, and there are no specific biomarkers to confirm AMI[20]. CTA imaging technology has officially replaced angiography as the imaging examination of choice, with a sensitivity of 93%, a specificity of 100%, a positive rate of 94%, and an exclusion rate of 100%[21].
Currently, the "4R" treatment strategy (resuscitation, rapid diagnosis, revascularization, and reassessment of bowel) is recommended internationally for the treatment of AMI[22]. In patients with early acute arterial embolism or thrombosis, the onset of intestinal ischemia is < 12 h, intestinal injury is usually in the reversible stage without obvious peritoneal irritation, and the lesions are located in the main trunk or branches. Endovascular catheter therapy combined with systemic anticoagulation can be the first choice for treatment[17,23]. In this case, the patient had acute abdominal pain, high blood pressure, and high-risk factors for dehydration, and the plasma D-dimer levels increased sharply.
After acute intra-abdominal multiple artery emboli were confirmed, although it was impossible to know whether there was bacterial embolism, timely intracatheter contact thrombolysis, percutaneous thrombosis aspiration, and systemic anticoagulation therapy were performed on the basis of strong anti-infection treatment, which avoided intestinal necrosis and diffuse peritonitis and improved the patient’s prognosis. Although hvKp infection can cause sepsis, the risk of infective endocarditis is very low[1], and the patient had no history of atrial fibrillation, which does not support mesenteric artery embolism due to shedding of infective endocarditis bacterial emboli.
Therefore, we speculated that hvKp entered his bloodstream through the damaged and exposed skin and then circulated to the mesenteric artery. Due to sepsis, high fever, and dehydration, the patient developed a hypercoagulable state, which resulted in the formation of bacterial emboli and thrombi. At present, the efficacy and safety of arterial thrombolysis are mainly reported for acute ischemic stroke secondary to infective endocarditis. Previously, infective endocarditis represented a classical contraindication to thrombolysis for acute ischemic stroke due to a potential increased risk of intracranial hemorrhage. However, some case reports have suggested the safety and potential efficacy of intravenous or intra-arterial thrombolysis in stroke related to infective endocarditis[24-27]. Notably, McCollom and Zwirko[28] reported a case of infected iliofemoral deep venous thrombosis that was successfully treated with catheter-directed thrombolysis, angioplasty, and stent placement. Although there are no reports of bacterial thrombolysis in mesenteric arteries, this case demonstrates that thrombolysis can be accomplished safely with adequate antibiotic coverage.
At present, community-acquired severe pneumonia complicated with acute intra-abdominal multiple arterial thrombosis and bacterial embolism caused by hvKp has not been reported in the literature, and there is no rapid and reliable clinical test to identify hvKp. In summary, we suggest that clinicians should consider the possibility of Gram-negative bacilli infection and conduct effective pathogen detection in a timely fashion, such as BALF or tissue mNGS, when managing patients with severe community-acquired pneumonia without waiting for bacteriologic and drug sensitivity results. At the same time, in cases of acute abdominal multiple artery embolism and organ dysfunction, thrombolysis and systemic anticoagulation need to be applied along with vascular radiologic intervention. Although thrombolytic therapy for the arterial system complicated with infectious bacteria thrombolysis is currently controversial, we believe that local thrombolytic therapy is still effective under effective anti-infection therapy; however, further research is warranted in the future.
We acknowledge the contributions of Mr. Xiu-Qing Liao, who endorsed the data and conclusions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saki M, Iran; Shelat VG, Singapore; Velikova TV, Bulgaria S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 463] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 2. | Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, Chan EW, Shu L, Yu J, Zhang R, Chen S. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 555] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 3. | Sánchez-López J, García-Caballero A, Navarro-San Francisco C, Quereda C, Ruiz-Garbajosa P, Navas E, Dronda F, Morosini MI, Cantón R, Diez-Aguilar M. Hypermucoviscous Klebsiella pneumoniae: A challenge in community acquired infection. IDCases. 2019;17:e00547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Altintas Ü, Lawaetz M, Riazi H, de la Motte L, Lindh M, Lönn L, Sillesen H, Eiberg J. Early- and Long-term Outcome After Endovascular Treatment of Chronic and Acute on Chronic Mesenteric Ischemia in a Large National Cohort. Eur J Vasc Endovasc Surg. 58:e631-e632. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lai YC, Lu MC, Hsueh PR. Hypervirulence and carbapenem resistance: two distinct evolutionary directions that led high-risk Klebsiella pneumoniae clones to epidemic success. Expert Rev Mol Diagn. 2019;19:825-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Hao Z, Duan J, Liu L, Shen X, Yu J, Guo Y, Wang L, Yu F. Prevalence of Community-Acquired, Hypervirulent Klebsiella pneumoniae Isolates in Wenzhou, China. Microb Drug Resist. 2020;26:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Rodrigues C, Passet V, Rakotondrasoa A, Diallo TA, Criscuolo A, Brisse S. Description of Klebsiella africanensis sp. nov., Klebsiella variicola subsp. tropicalensis subsp. nov. and Klebsiella variicola subsp. variicola subsp. nov. Res Microbiol. 2019;170:165-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Liu C, Guo J. Hypervirulent Klebsiella pneumoniae (hypermucoviscous and aerobactin positive) infection over 6 years in the elderly in China: antimicrobial resistance patterns, molecular epidemiology and risk factor. Ann Clin Microbiol Antimicrob. 2019;18:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Palacios M, Miner TA, Frederick DR, Sepulveda VE, Quinn JD, Walker KA, Miller VL. Identification of Two Regulators of Virulence That Are Conserved in Klebsiella pneumoniae Classical and Hypervirulent Strains. mBio. 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Evangelista V, Gonçalves CV, Almeida R, Henriques C, Baptista AM, da Graça JP, Araújo JL. Klebsiella pneumoniae Invasive Syndrome. Eur J Case Rep Intern Med. 2018;5:000800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Morikawa K, Okada F, Ando Y, Ishii R, Matsushita S, Ono A, Maeda T, Mori H, Yamashita S, Kawahara K. Meticillin-resistant Staphylococcus aureus and meticillin-susceptible S. aureus pneumonia: comparison of clinical and thin-section CT findings. Br J Radiol. 2012;85:e168-e175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45:668-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 13. | Khaertynov KS, Anokhin VA, Rizvanov AA, Davidyuk YN, Semyenova DR, Lubin SA, Skvortsova NN. Virulence Factors and Antibiotic Resistance of Klebsiella pneumoniae Strains Isolated From Neonates With Sepsis. Front Med (Lausanne). 2018;5:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Liu C, Du P, Xiao N, Ji F, Russo TA, Guo J. Hypervirulent Klebsiella pneumoniae is emerging as an increasingly prevalent K. pneumoniae pathotype responsible for nosocomial and healthcare-associated infections in Beijing, China. Virulence. 2020;11:1215-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Harada S, Aoki K, Yamamoto S, Ishii Y, Sekiya N, Kurai H, Furukawa K, Doi A, Tochitani K, Kubo K, Yamaguchi Y, Narita M, Kamiyama S, Suzuki J, Fukuchi T, Gu Y, Okinaka K, Shiiki S, Hayakawa K, Tachikawa N, Kasahara K, Nakamura T, Yokota K, Komatsu M, Takamiya M, Tateda K, Doi Y. Clinical and Molecular Characteristics of Klebsiella pneumoniae Isolates Causing Bloodstream Infections in Japan: Occurrence of Hypervirulent Infections in Health Care. J Clin Microbiol. 2019;57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Salsano A, Salsano G, Spinella G, Palombo D, Santini F. Acute Mesenteric Ischemia: Have the Guidelines of the World Society of Emergency Surgery Analyzed All the Available Evidence? Cardiovasc Intervent Radiol. 2018;41:358-359. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Kerzmann A, Haumann A, Boesmans E, Detry O, Defraigne JO. [Acute mesenteric ischemia]. Rev Med Liege. 2018;73:300-303. [PubMed] [Cited in This Article: ] |
| 18. | Liao G, Chen S, Cao H, Wang W, Gao Q. Review: Acute superior mesenteric artery embolism: A vascular emergency cannot be ignored by physicians. Medicine (Baltimore). 2019;98:e14446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Pay L, Kolak Z, Çakır B, Kamber T, Yazıcı S. Atrial fibrillation-related acute myocardial infarction and acute mesenteric ischemia. Turk Kardiyol Dern Ars. 2021;49:410-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 20. | Salsano G, Salsano A, Sportelli E, Petrocelli F, Dahmane M, Spinella G, Pane B, Mambrini S, Palombo D, Santini F. What is the Best Revascularization Strategy for Acute Occlusive Arterial Mesenteric Ischemia: Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol. 2018;41:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Obmann MM, Punjabi G, Obmann VC, Boll DT, Heye T, Benz MR, Yeh BM. Dual-energy CT of acute bowel ischemia. Abdom Radiol (NY). 2022;47:1660-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Ben Abdallah I, Castier Y, Corcos O. [Mesenteric arterial ischemia: from diagnosis to decision]. Rev Prat. 2021;71:853-859. [PubMed] [Cited in This Article: ] |
| 23. | Altintas Ü, Lawaetz M, de la Motte L, Riazi H, Lönn L, Lindh M, Sillesen H, Eiberg J. Endovascular Treatment of Chronic and Acute on Chronic Mesenteric Ischaemia: Results From a National Cohort of 245 Cases. Eur J Vasc Endovasc Surg. 2021;61:603-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Siccoli M, Benninger D, Schuknecht B, Jenni R, Valavanis A, Bassetti C. Successful intra-arterial thrombolysis in basilar thrombosis secondary to infectious endocarditis. Cerebrovasc Dis. 2003;16:295-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Junna M, Lin CC, Espinosa RE, Rabinstein AA. Successful intravenous thrombolysis in ischemic stroke caused by infective endocarditis. Neurocrit Care. 2007;6:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tan M, Armstrong D, Birken C, Bitnun A, Caldarone CA, Cox P, Kahr W, Macgregor D, Askalan R. Bacterial endocarditis in a child presenting with acute arterial ischemic stroke: should thrombolytic therapy be absolutely contraindicated? Dev Med Child Neurol. 2009;51:151-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Sontineni SP, Mooss AN, Andukuri VG, Schima SM, Esterbrooks D. Effectiveness of Thrombolytic Therapy in Acute Embolic Stroke due to Infective Endocarditis. Stroke Res Treat. 2010;2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | McCollom VE, Zwirko RM. Catheter-directed thrombolysis in acute, infected iliofemoral venous thrombosis. J Vasc Interv Radiol. 1998;9:941-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |