03 September 2021: Clinical Research
Genital Human Papillomavirus Prevalence and Genotyping Among Males in Putuo District of Shanghai, China 2015–2019
Xiaoxiao Li1ACDEG*, Fenfen Xiang1C, Zixi Chen1D, Tao Zhang1B, Zhaowei Zhu1BC, Mengzhe Zhang1BF, Rong Wu1DG, Xiangdong Kang1ADOI: 10.12659/MSM.932093
Med Sci Monit 2021; 27:e932093
Abstract
BACKGROUND: Reports of human papillomavirus (HPV) infection and genotype distribution in Chinese men are limited, and HPV vaccination has not yet been recommended for men in China.
MATERIAL AND METHODS: We retrospectively reviewed the prevalence and genotyping of male genital HPV. A total of 1227 male patients (aged 17 to 81 years) attending the dermatology and sexually transmitted disease clinics at Putuo District Center Hospital in Shanghai from 2015 to 2019 were included. Genital exfoliated specimens were obtained for detection and genotyping of 27 HPV types by Luminex-based multiplex assay.
RESULTS: The prevalence of any HPV was 65.5% (804/1227). The rate of multiple infection was 25.8% (317/1227). The 5 main HPV types were 6 (32.0%), 11 (23.2%), 16 (5.6%), 43 (4.3%), and 59 (4.0%). Among all detected HPV genotypes, 65.5% (875/1336) were 9-valent HPV genotypes. No significant differences were observed in the detection rate of HPV infection over 5 years (P>0.05). Age groups ≤24 years (70.7%) and ≥55 years (72.9%) showed higher infection rates, and significant differences were detected in rates of low-risk HPV infection in different age-stratified groups (P<0.05). Prevalence of HPV infection among patients with warts (74.4%) was significantly higher than that of patients with other clinical characteristics (40.4%) and physical examination (63.6%).
CONCLUSIONS: Our study suggested that more than half of Chinese male patients have detectable HPV infections, and penis-genital and anogenital warts were the most common clinical manifestations. Moreover, the available 9-valent HPV vaccine covers the most frequently observed HPV types among men.
Keywords: Genotype, Papillomaviridae, Papillomavirus Vaccines, Prevalence, Adolescent, Aged, 80 and over, Cross-Sectional Studies, Genital Diseases, Male, Humans, Papillomavirus Infections, young adult
Background
Human papillomavirus (HPV) is one of most common sexually transmitted viruses in men and women worldwide and can cause many types of dermatology problems and diseases, from benign warts to various cancers [1,2]. Several sexually transmitted infectious diseases, including HIV and HSV-2, are strongly correlated with HPV infection [3].
Each year, there are nearly 34 800 new HPV-related cancer cases in the United States, with 13 100 cases in men. Of these cases, 92% are caused by infection with the HPV genotype, which is covered by the 9-valent HPV vaccine [17]. Currently, there are 3 HPV vaccines that can protect against multiple HPV genotypes: bivalent (2v, HPV-16/18), quadrivalent (4v, HPV-6/11/16/18), and 9-valent (9v, HPV-6/11/16/18/31/33/45/52/58) vaccines [18]. HPV vaccinations have a great preventive potential to reduce HPV-associated diseases in men and women [19–22]. However, most countries, including China, have only administered vaccines to female patients but not to males [23]. It has been reported that 71 countries (37%) provided HPV vaccines for females, while only 11 countries (6%) provided vaccines for males in 2017 [24]. Comprehensive studies of male HPV infection and types are crucially important for developing evidence-based HPV vaccination, while relevant information in Chinese men is still scarce [25].
The HPV infection rate is an important parameter when considering the cost-effectiveness of HPV vaccines. In addition, regional differences are essential in the distribution of male HPV, and age can be the underlying cause affecting the incidence of HPV genotypes [26]. This study aimed to assess HPV infection prevalence and genotype distribution among Chinese male patients (Shanghai) who visted our dermatology and sexually transmitted disease (STD) clinics from 2015 to 2019. To improve the understanding of HPV infection and types in Chinese men, an age-stratified analysis and theoretical vaccine coverage analysis were performed.
Material and Methods
STUDY POPULATION:
This retrospective cross-sectional study was conducted from January 1, 2015 to December 31, 2019. A total of 1227 male patients attending a dermatology department and STD clinic in Putuo District Center Hospital were included in this study. The inclusion criteria were as follows: (1) male patients; (2) patients ≥17 years old; (3) patients with signs such as anogenital wart and condyloma acuminata; (4) clinical manifestations of urethritis, rash, and dermatitis; (5) patients with discomfort or who had undergone physical examination; (6) willingness to provide adequate genital samples for the HPV DNA genotyping test; and (7) agreement to participate in our study. This study was performed in accordance with the principles of Declaration of Helsinki and was approved by the Ethics Committee of the Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (Shanghai, China) (reference no. PTEC-A-2020-24-2). Written informed consent was obtained from each patient at a previous clinical visit. To protect personal privacy, data were deidentified before analysis.
HPV SAMPLE COLLECTION AND DNA EXTRACTION:
Genital scraped cells from the urethral epithelium, anus, or penile epidermis were collected by physicians with a nylon flocked swab. The swab was placed in separate 3-mL tubes containing transport medium and immediately transported to a clinical laboratory affiliated with our hospital for the HPV testing. Specimens that could not be shipped for the examination in time were stored at 4°C and analyzed within 72 h. A total of 200 μL of the specimen mixture was transferred to a new 1.5-mL centrifuge tube and centrifuged at 12 000 rpm for 10 min. The precipitate was retained, and 200 μL of exfoliated cell nucleic acid release agent (Tellgen Life Science Co. Ltd. Shanghai, China) was added, followed by incubation for 15 min at 100°C with a metal bath. Afterward, the Eppendorf tube was centrifuged at 12 000 rpm for 5 min, and the supernatant was kept and moved to a new Eppendorf tube for polymerase chain reaction (PCR) examination or stored at −20°C.
PCR AMPLIFICATION AND HYBRIDIZATION:
A Tellgenplex™ HPV DNA genotyping test assay kit (Shanghai Tellgen Life Science, Co. Ltd.) was used for detection and genotyping 27 HPV types, including 19 HR-HPV genotypes (HPV-16/18/26/31/33/35/39/45/51/52/53/55/56/58/59/66/68/82/83) and 8 LR-HPV genotypes (HPV-6/11/40/42/43/44/61/81) by a combination of PCR and Luminex technology [26]. The detailed experimental operation protocol is included in previous studies [27,28]. In brief, PCR testing was performed in a total amplification volume of 20 μL/reaction per sample, containing 15 μL of the reaction solution (including 10 μL master mix, 5.0 μL primers, and 0.8 μL taq DNA polymerase) and 5 μL of the extracted DNA, according the manufacturer’s instructions. The PCR reaction condition was as follows: 95°C for 5 min, 1 cycle; 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, 5 cycles; 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, total of 35 cycles; and 72°C for 3 min, 1 cycle. After amplification, 3 μL of the PCR product and 22 μL of hybridization solution (containing carboxylated beads coupled with oligonucleotide probes for each of the 27 genotypes) were added into each well of a 96-well plate. Subsequently, hybridization was conducted at 95°C for 5 min followed by 48°C for 30 min. The hybridization product was stained by streptavidin-R-phycoerythrin (75 μL) and incubated at 48°C for 15 min. Finally, the products were analyzed by a Luminex 200 bioanalyzer. The human β-actin gene was used as an internal control and assessed for each specimen to ensure that each tube contained enough cellular components for the HPV genotyping. The positive and negative contols in the reaction were HPV-16/18 genotype plasmid and ultrapure water, respectively.
STATISTICAL ANALYSIS:
Excel 2010 and SPSS version 22.0 (IBM Corp, Armonk, NY, USA) were used to collate and analyze the data, respectively. Figures were drawn with GraphPad Prism 6.02. We calculated the overall and specific genotype prevalences of HPV infection. A binomial 95% confidence interval (CI) was also estimated for calculation of the prevalence of each type of HPV. The distribution of categorical variables in different groups was evaluated by using the chi-squared test. Also, the positive infection rate of studied participants with different clinical characteristics was also calculated. The patients were stratified by age: ≤24, 25–34, 35–44, 45–54, and ≥55 years. The gamma value and linear-by-linear association test were used to evaluate the trend changes in HPV infection rates by year and age. A
Results
GENITAL HPV PREVALENCE AND TRENDS:
In our study, 804 of the 1227 patients examined were infected with at least 1 genotype of HPV; thus, the prevalence of HPV infection was 65.5%. Specifically, the rate of HPV infection in patients with only 1 genotype was 39.69% (487/1227), and the percentage of patients with multiple HPV infections (≥2 genotypes) was 25.84% (317/1227) (Table 1, Figure 1). Further analysis indicated that 15.57% (191/1227) of patients had double infections, 5.79% (71/1227) had triple infections, and 3.02% (37/1227) had quadruple infections. The detection of single high-risk types and low-risk types accounted for 5.46% (67/1227) and 33.33% (409/1227), respectively. Among the mixed risk types, the detection rate of patients with 2 or more HR-HPV genotypes was 2.44% (30/1227), the multiple LR-HPV genotypes detection rate was 4.16% (51/1227), and 20.13% (247/1227) of patients had a mixture of HPV infection containing HR-HPV and LR-HPV genotypes (Table 1). In terms of the linear-by-linear association test, no significant difference was found in the rates of HPV infection from 2015 to 2019 (P>0.05) (Table 2, Figure 1). Interestingly, the prevalence of HR-HPV genotypes had a statistically significant decreasing trend from 2015 to 2019 (P=0.001), while no significant difference was observed in the detection rates of LR-HPV genotypes over this period (P>0.05) (Table 2).
DISTRIBUTION AND GENOTYPES OF MALE HPV INFECTION:
Overall, the rate of any HPV genotype infection was 65.5% (95% CI, 62.9–68.2%), ranging from 60.9% to 70.1%. Among the HPV-positive patients, the rate of HR-HPV infection was 28.0% (95% CI, 25.5–30.5%), and the LR-HPV genotype infection rate was 57.6% (95% CI, 54.9–60.4%). The 5 HR-HPV types with the highest detection rate among patients in the STD clinic were HPV-16 (5.6%), HPV-59 (4.0%), HPV-52 (3.4%), HPV-51 (3.3%), and HPV-56 (3.1%) (Figure 2). In addition, the 3 most frequently observed LR-HPV types were HPV-6 (32.0%), HPV-11 (23.2%), and HPV-43 (4.3%) (Table 2, Figure 2).
HPV GENOTYPE INFECTION IN DIFFERENT AGE GROUPS:
The HPV infection rates of age groups ≤24, 25–34, 35–44, 45–54, and ≥55 years were 70.75% (116/164), 63.25% (315/498), 58.73% (148/252), 70.45% (93/132), and 72.93% (132/181), respectively. Figure 3 shows that the infection rate of the ≤24 years group exhibited a peak, with an age-specific prevalence of total HPV infection at ≤24 years old. Then, the infection rate sharply dropped with increasing age and finally surged again in patients over 45 years old. The pattern of the age-specific LR-HPV infection rate was similar to that of any type of infection and showed an increasing trend with age (P<0.05). However, HR-HPV infection exhibited a relatively flat curve (P>0.05) (Figure 3). There were significant differences in the detection rate of the single LR-HPV type among the classified age groups (HPV-6, P<0.05) and the 4 HR-HPV types (HPV-16, -52, -53, and -59, P<0.05) (Table 3). The prevalence of HPV infection exhibited a downward trend in the age group 25–34 years and an upward trend in the age group ≥55 years over the investigated 5 years (Table 4).
COVERAGE OF THE HPV INFECTION BY VACCINES:
In this study, we detected a total of 1336 HPV genotype numbers. Among the 1336 HPV genotypes, 480 (35.9%) were HR-HPV and 856 (64.1%) were LR-HPV. The total number of detected genotypes targeted by 2v, 4v, and 9v vaccines were 99 (7.4%), 777 (58.2%), and 875 (65.5%), respectively. All the detected 9v, non-9v HR-HPV, and non-9v LR-HPV genotypes and rates are shown in Figure 4. The prevalence by HPV type among patients by age group is shown in Table 5.
RELATIONSHIP OF HPV INFECTION AND CLINICAL SYMPTOMS:
By analyzing the patients’ files, we found that the clinical manifestations of HPV infection included penis-genital warts, anogenital warts, common warts, urethritis, rash, and dermatitis. Studied patients were divided into wart, physical examination, and “other” groups. The HPV infection rate of the wart group was 74.4% (360/484). More specifically, the positive rates of HPV infection in patients diagnosed with penis-genital warts, anogenital warts, and common warts were 78.9% (195/247), 81.0% (111/137), and 54.0% (54/100), respectively. It is worth noting that the prevalence of HPV infection in the physical examination group was 63.6% (378/594). The HPV detection rate of the “other” group, including urethritis (28.6%), rash (46.1%), and dermatitis (42.1%), was 40.4% (40/99) (Table 6).
Discussion
There are limited studies on HPV distribution among men from the Asia-Pacific region, with even fewer reports from China. To better control and reduce the prevalence of HPV infection, more comprehensive information on the distribution of HPV types and the effectiveness of vaccines in a targeted male group is needed in China. This study provides supplementary information on the distribution of genital HPV infection in Chinese male patients by year, age group, and HR-HPV and LR-HPV genotypes.
HPV is associated with a variety of diseases and is commonly detected in the oral cavity, urethra, anus, penis, and certain tumor tissues [29]. It has been reported that the average anal HPV positivity rate of any genotype among men who have sex with men in China is 62.1% [30]. The HPV infection rate of male outpatients with urethral or penile sampling sites in the Qingyuan area of Guangzhou is 54.3% [31]. In the present clinic-based study, we investigated the genotyping of the urethral epithelium, anus, or penile epidermis from 1227 male patients in the Putuo district, and the overall HPV infection rate was 65.5%. Our study was conducted in Shanghai, a large economically developed city, with a population of nearly 20 million and location in eastern China. We observed more than half of the men who visited our dermatology and STD clinic had at least 1 HPV genotype infection. The detection rate of genital HPV infection in our study population was higher than that of previous studies in Henan province (17.5%, from the general population) [32] and Beijing (50.9%, from an STD clinic) [12]. The different geographical areas of China, study cohorts (study population from clinic or general), and HPV testing methods might account for the observed heterogeneity. In addition, in one report, the prevalence of HPV among men varied greatly in different countries, ranging from 12.9% (Spain) to 80% (Brazil) [33]. However, Sudenga et al indicated that the infection rates of any genital HPV type in men from Brazil, Mexico, and the United States were 60.2%, 49.2%, and 46.9%, respectively [34]. Recently, Matsuzawa et al revealed that the percentage of any HPV infection among Japanese men who visited the urological clinics is 24.8% [35]. The rate was much lower than our results because our study population of STD clinic outpatients may have higher exposure associated with high-risk sexual behaviors.
In our study, a total of 344 (28.0%) cases were high-risk infection, and 707 (57.6%) cases were low-risk infection. Single infections are the most common manifestation of LR-HPV infections, and LR-HPV infections are more common than HR-HPV infections. The most frequently observed LR-HPV types are HPV-6 and HPV-11. They are the predominant types that can cause condyloma acuminata in all countries [36,37]. In the present study, HPV types 16, 59, 52, and 51 were the top 4 HR-HPV types reported, while results from another study conducted in southern China showed that HPV types 16, 6, 52, and 58 are the most frequent genotypes among male homosexual participants [23]. The dominant types found in the cervical region of Chinese women are HPV types 16, 18, 52, and 58 [38]. HPV-16 and HPV-52 are the most frequently encountered types in men and women, which is consistent with our observation. Meanwhile, it was worth noting that HPV-59 and HPV-51 were also the most commonly observed HR-HPV types in the present study. Since they are still not covered in the latest 9v HPV vaccine, these 2 high-risk types should attract the attention of researchers who are developing HPV vaccines.The 9v HPV vaccine covers nearly 65.5% of the detected genotypes in this study, which means that the vaccine may be effective in preventing HPV infection in Chinese men. Men infected with HPV are usually unfamiliar with the disease and transmit it to their sexual partners, which would increase the risk of HPV-associated diseases, including tumors. Therefore, regular pathological examination, follow-up, and strengthened treatment of infected patients should be recommended.
Different age groups can exhibit different rates and main types of HPV infection. Several studies found that the age-specific rate of any type of infections among women formed a quasi-U-shaped curve with 2 peaks in groups aged ≤24 years and ≥55 years [39,40]. One theory is that young women may have less sensitive immune systems and are more likely to have frequent sexual activity. The second peak in the older group may be due to the decline in the immune function of women in this age group and virus clearance ability, leading to an increase in HPV infection. In the present study, the HPV infection rates of the groups aged ≤24 years and ≥55 years were 70.75% and 72.93%, respectively, which were higher than those of other age groups. Therefore, the relationship between age and HPV infection rate in men in our study was similar to the pattern in women found in other studies. The prevalence of HPV in the age group ≥55 years showed an upward trend from 2015 to 2019. Considering that older men with persistent HPV infection are at high risk of anal and penile cancer, and a certain percentage of men do develop HPV-associated diseases, it is necessary and beneficial to perform comprehensive and regular screening for older men with HPV infection [34]. In addition, our results also suggested that young men (≤24 years) were more susceptible to HPV infection.
Penis-genital and anogenital warts are also called condyloma acuminata. Similar to a previous study [31], the HPV detection rate was highest in patients with the clinical manifestiation of condyloma acuminata in the present study. The main objective of genital treatment is the destruction and removal of the lesions, as well as local modification. Currently, there is no specific and established therapy to eradicate the HPV virus, but vaccines can decrease the incidence of certain HPV infections. Treatment strategies for condyloma acuminata have been summarized by Fathi et al, including combination and monotherapies [41]. It has been reported that imiquimod 3.75% medicated cream has been approved for treating anogenital warts in patients aged ≥12 years [42]. We also noticed that the positive rate of HPV infection in the population without obvious symptoms reached 63.6%. Vaccination and cervical screening have been used for the primary prevention of HPV infection in women [43]. However, whether a screening method would be also applicable to men needs further research and discussion. On the other hand, strategies and policies toward the mitigation of HPV infection, such as vaccination, should target both females and males.
There are some limitations to keep in mind when interpreting these study results. First, the results may not represent the general male population because the study included only those patients who attended the dermatology and STD clinic and who were tested for HPV. Thus, the reported HPV infection rate might be higher than that of the general population and cannot be representative of all males in Shanghai. Second, this was a single-center pilot study including data only from one hospital. Third, the inability to obtain complete information about the sociodemographic and behavioral characteristics of recruited men and the lack of such information make it difficult to assess the effect of specific patient characteristics on HPV infection.
Conclusions
In conclusion, this study investigated the status of genital HPV infection among men from an STD clinic in Shanghai, China, from 2015 to 2019. Results revealed the percentage of male HPV infection was high. Moreover, it also showed that HPV types 16, 59, 52, 51, and 56 were the dominant HR-HPV types, and the most frequently observed LR-HPV genotypes were HPV types 6, 11, and 43. Since June 2016, vaccinations have been recommended for females aged 9 to 45 years in mainland China [44]. Molecular epidemiological studies and targeted HPV vaccination among high-risk Chinese males are urgently needed.
Figures
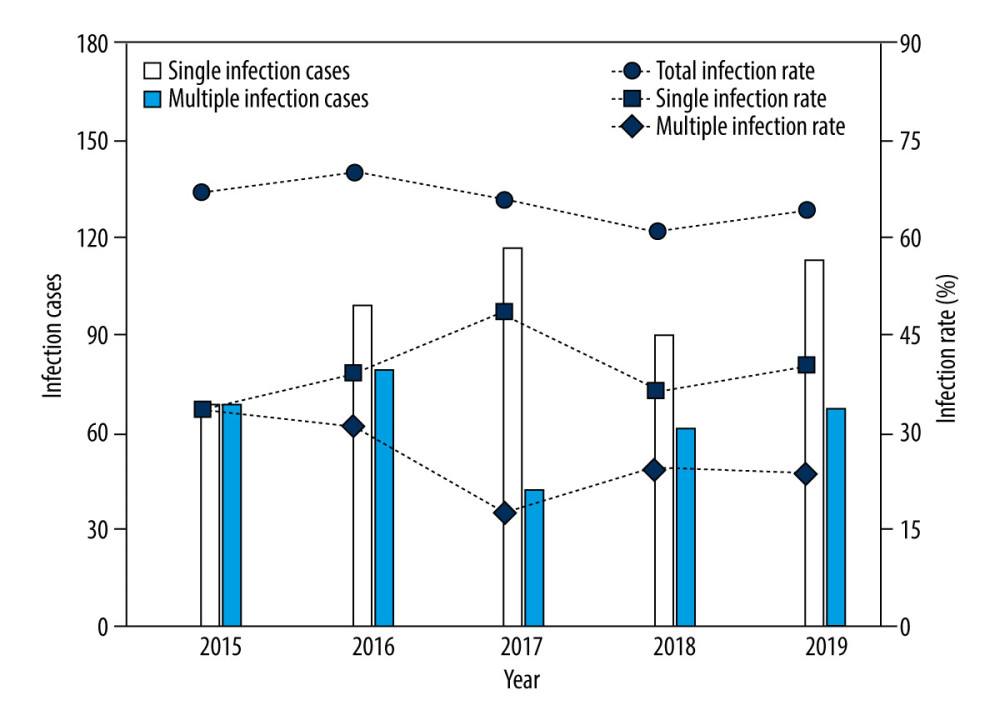 Figure 1. Changes in the infection cases and infection rates of human papillomavirus (HPV) in male patients who visted our sexually transmitted disease (STD) clinic over 2015 to 2019.
Figure 1. Changes in the infection cases and infection rates of human papillomavirus (HPV) in male patients who visted our sexually transmitted disease (STD) clinic over 2015 to 2019. 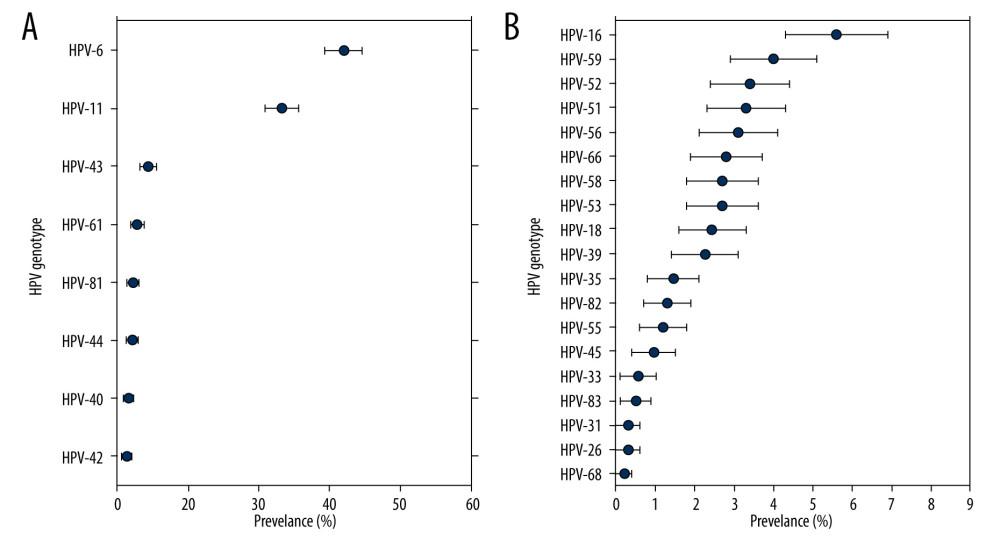 Figure 2. Type-specific prevalence of HPV among men in sexually transmitted disease (STD) clinic, 2015–2019. (A) LR-HPV types; (B) HR-HPV types. Vertical bars represent 95% confidence intervals.
Figure 2. Type-specific prevalence of HPV among men in sexually transmitted disease (STD) clinic, 2015–2019. (A) LR-HPV types; (B) HR-HPV types. Vertical bars represent 95% confidence intervals. 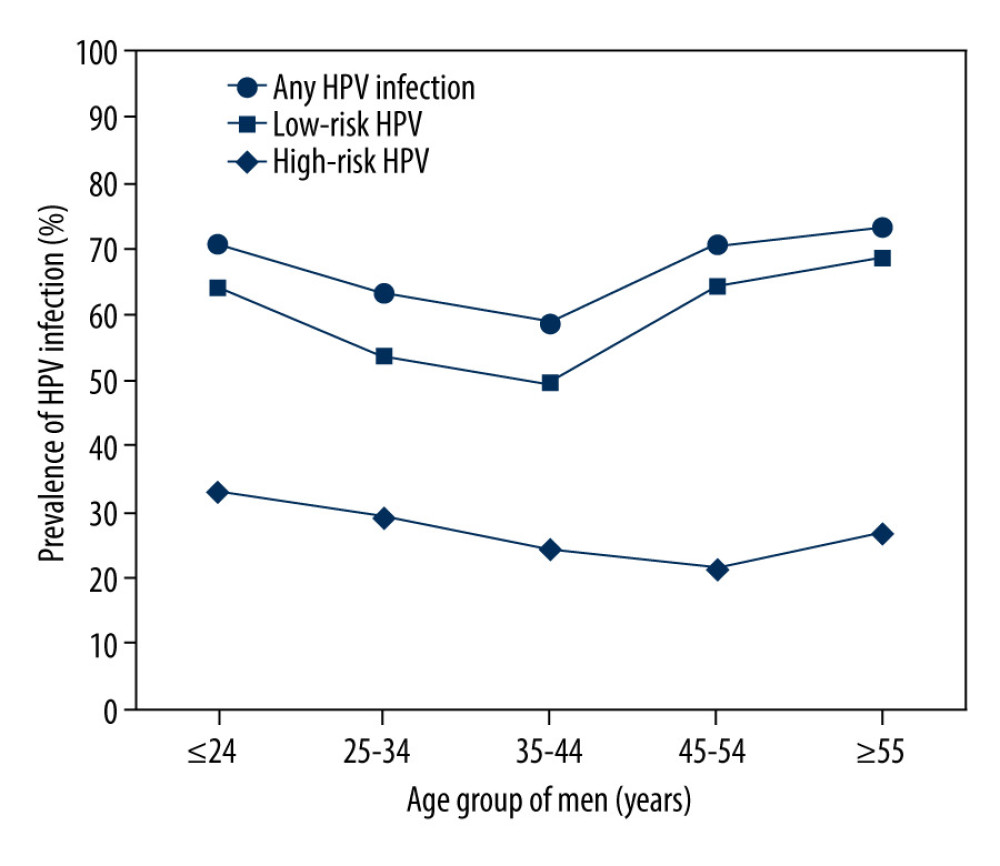 Figure 3. Prevalence of HR, low-risk(LR) and any HPV infection in different age groups.
Figure 3. Prevalence of HR, low-risk(LR) and any HPV infection in different age groups. 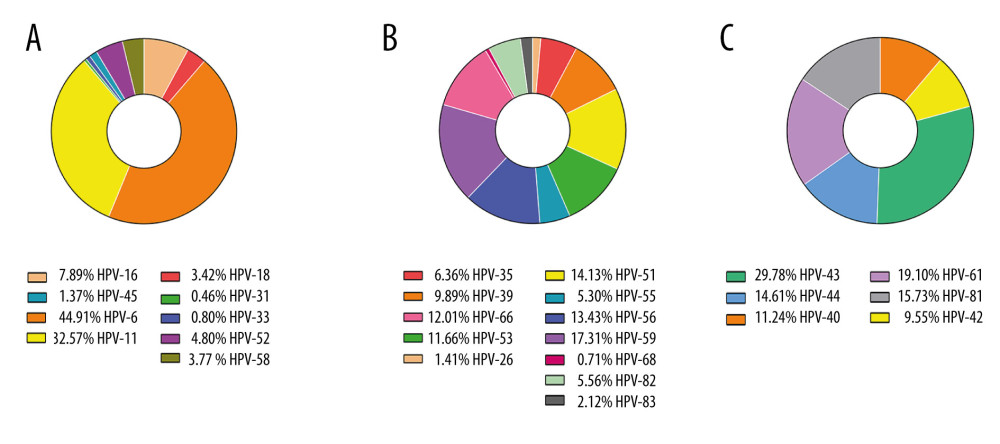 Figure 4. The detection rates among 9v HPV genotypes (A), non-9v HR-HPV genotypes (B) and non-9v LR-HPV genotypes (C).
Figure 4. The detection rates among 9v HPV genotypes (A), non-9v HR-HPV genotypes (B) and non-9v LR-HPV genotypes (C). Tables
Table 1. Detection of HPV genotypes of male patients in a sexually transmitted disease clinic from 2015 to 2019.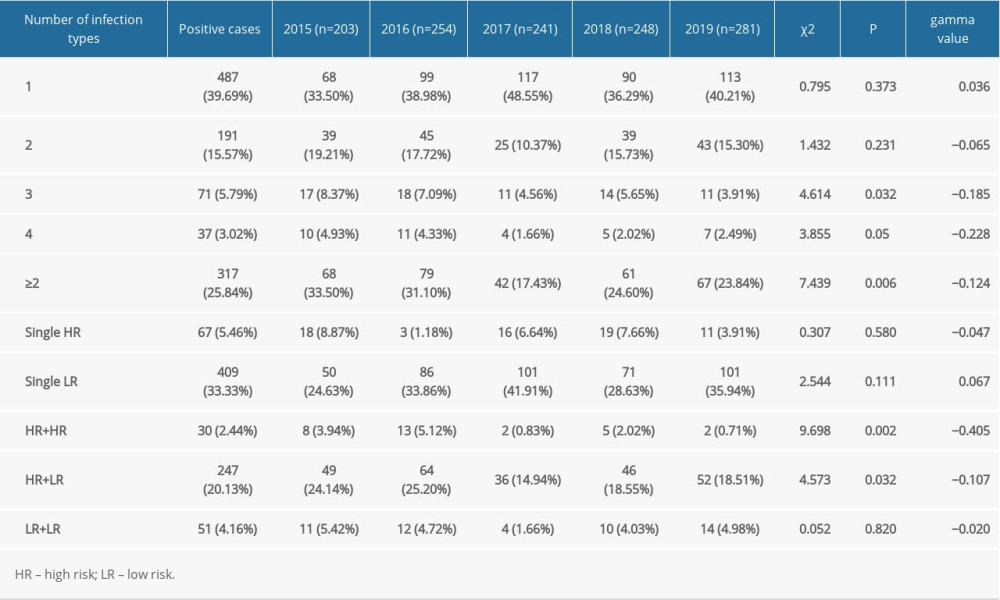 Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019.
Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019.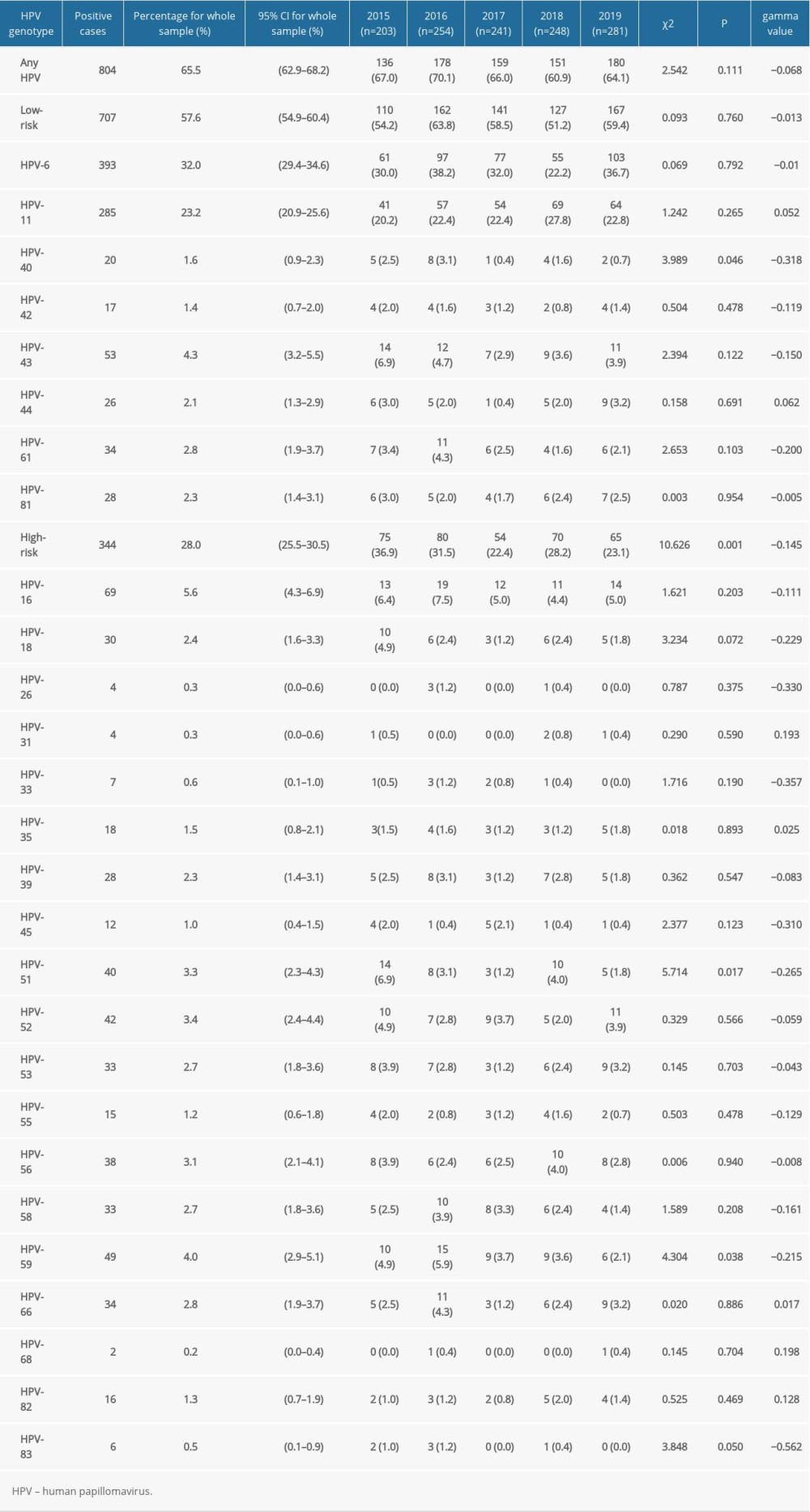 Table 3. Changes of type-specific genital human papillomavirus detection across different age groups.
Table 3. Changes of type-specific genital human papillomavirus detection across different age groups.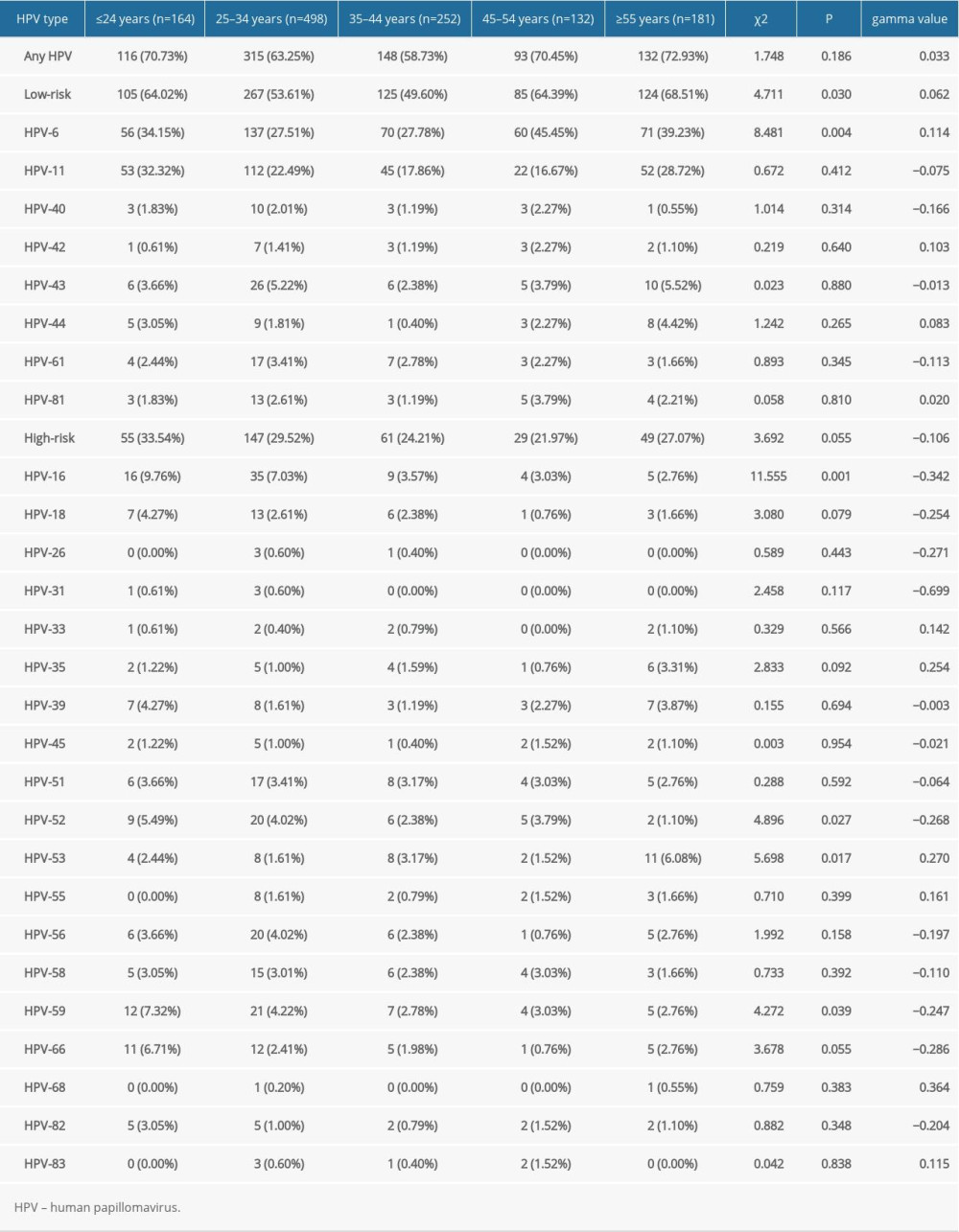 Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019.
Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019.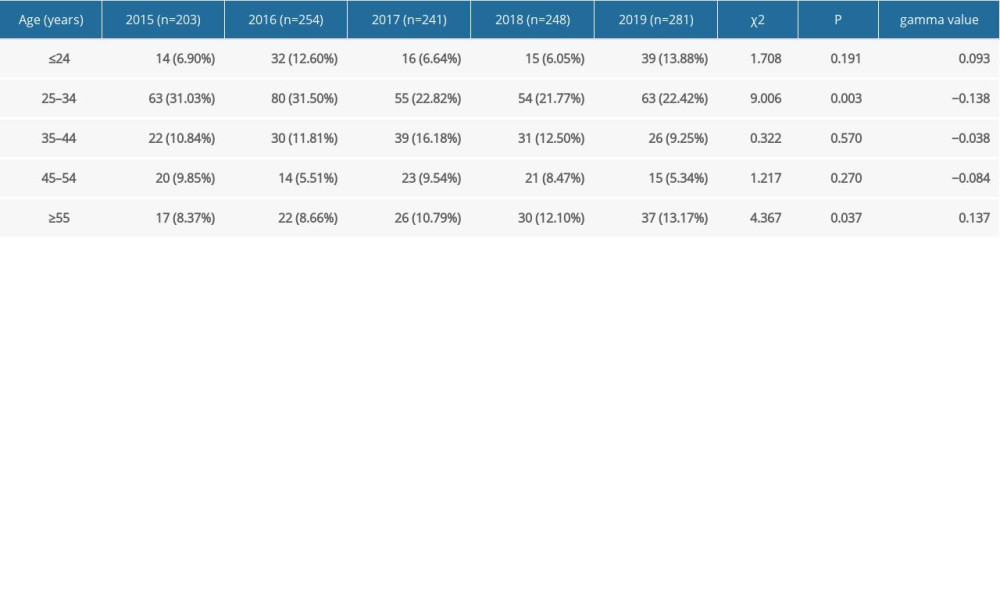 Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years.
Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years.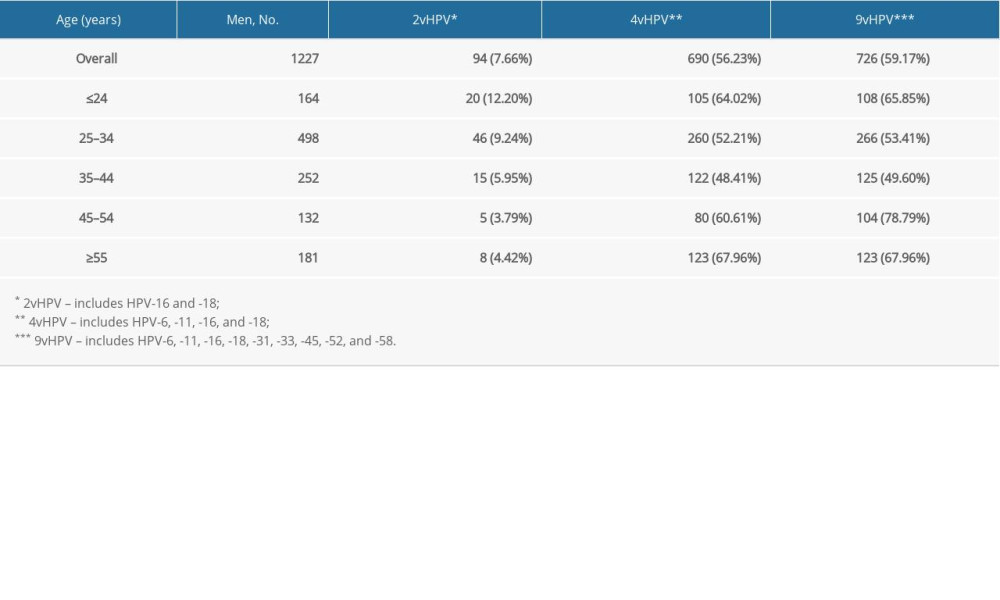 Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types.
Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types.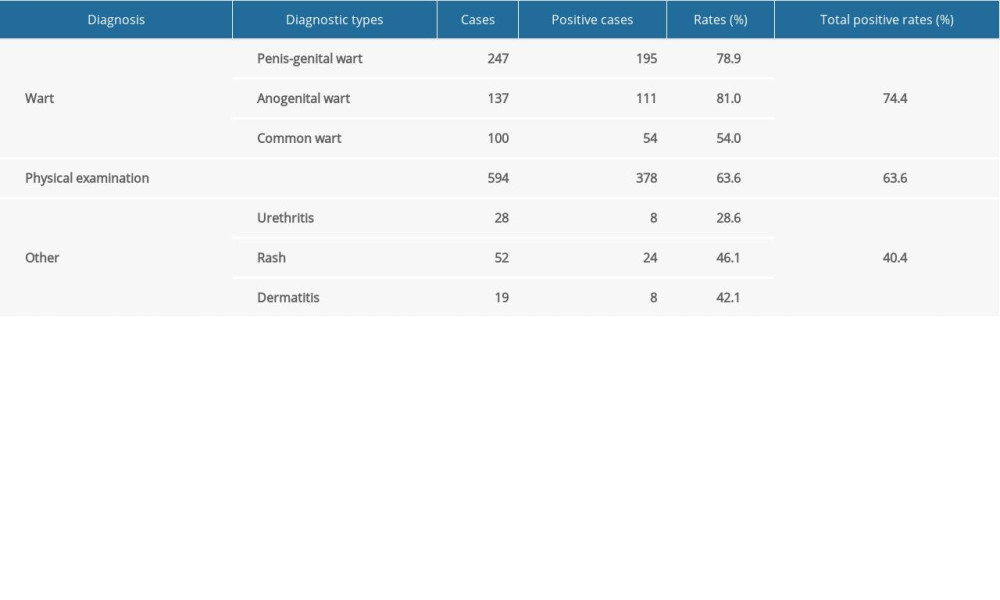
References
1. Braaten KP, Laufer MR, Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine: Rev Obstet Gynecol, 2008; 1(1); 2
2. Liu X, Lin H, Chen X, Prevalence and genotypes of anal human papillomavirus infection among HIV-positive vs. HIV-negative men in Taizhou, China: Epidemiol Infect, 2019; 147; e117
3. Taku O, Brink A, Meiring TL, Detection of sexually transmitted pathogens and co-infection with human papillomavirus in women residing in rural Eastern Cape, South Africa: Peer J, 2021; 9; e10793
4. Di Pietro M, Filardo S, Porpora MG, HPV/Chlamydia trachomatis co-infection: Metagenomic analysis of cervical microbiota in asymptomatic women: New Microbiol, 2018; 41(1); 34-41
5. Lin C-C, Hsieh M-C, Hung H-C, Human papillomavirus prevalence and behavioral risk factors among HIV-infected and HIV-uninfected men who have sex with men in Taiwan: Medicine, 2018; 97(45); e13201
6. Zur Hausen H, Papillomaviruses and cancer: From basic studies to clinical application: Nat Rev Cancer, 2002; 2(5); 342-50
7. Muñoz N, Bosch FX, De Sanjosé S, Epidemiologic classification of human papillomavirus types associated with cervical cancer: New Engl J Med, 2003; 348(6); 518-27
8. Akogbe GO, Ajidahun A, Sirak B, Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States: Int J Cancer, 2012; 131(3); E282-91
9. Backes DM, Kurman RJ, Pimenta JM, Smith JS, Systematic review of human papillomavirus prevalence in invasive penile cancer: Cancer Causes Control, 2009; 20(4); 449-57
10. Bzhalava Z, Mühr LSA, Dillner J, Transcription of human papillomavirus oncogenes in head and neck squamous cell carcinomas: Vaccine, 2020; 38(25); 4066-70
11. Martel CD, Plummer M, Vignat J, Franceschi S, Worldwide burden of cancer attributable to HPV by site, country and HPV type: Int J Cancer, 2017; 141(4); 664-70
12. Xin H, Li H, Li Z, Genital HPV infection among heterosexual and homosexual male attendees of sexually transmitted diseases clinic in Beijing, China: Epidemiol Infect, 2017; 145(13); 2838-47
13. Hernandez AL, Efird JT, Holly EA, Incidence of and risk factors for type-specific anal human papillomavirus infection among HIV-positive MSM: AIDS (London, England), 2014; 28(9); 1341
14. Lin C, Franceschi S, Clifford GM, Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis: Lancet Infect Dis, 2018; 18(2); 198-206
15. Olesen TB, Sand FL, Rasmussen CL, Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: A systematic review and meta-analysis: Lancet Oncol, 2019; 20(1); 145-58
16. Garland SM, Steben M, Sings HL, Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine: J Infect Dis, 2009; 199(6); 805-14
17. Senkomago V, Henley SJ, Thomas CC, Human papillomavirus – attributable cancers – United States, 2012–2016: MMWR-Morbid Mortal W, 2019; 68(33); 724
18. Jørgensen L, Doshi P, Gøtzsche P, Jefferson T, Challenges of independent assessment of potential harms of HPV vaccines: BMJ (Analysis), 2018; 362; k3694
19. Schmeler KM, Sturgis EM, Expanding the benefits of HPV vaccination to boys and men: Lancet, 2016; 387(10030); 1798
20. Giuliano AR, Palefsky JM, Goldstone S, Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males: New Engl J Med, 2011; 364(5); 401-11
21. Garland SM, Hernandez-Avila M, Wheeler CM, Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases: New Engl J Med, 2007; 356(19); 1928-43
22. Stanley M, HPV vaccination in boys and men: Hum Vacc Immunother, 2014; 10(7); 2109-11
23. Zhou Y, Lin YF, Meng X, Anal human papillomavirus among men who have sex with men in three metropolitan cities in southern China: Implications for HPV vaccination: Vaccine, 2020; 38(13); 2849-58
24. , Human papillomavirus vaccines: WHO position paper, May 2017: Wkly Epidemiol Rec, 2017; 92; 241-68
25. Patel P, Bush T, Conley L, Prevalence, incidence, and clearance of human papillomavirus types covered by current vaccines in men with human immunodeficiency virus in the SUN study: J Infect Dis, 2020; 222(2); 234-42
26. Poynten IM, Machalek D, Templeton D, Comparison of age-specific patterns of sexual behaviour and anal HPV prevalence in homosexual men with patterns in women: Sex Transm Infect, 2016; 92(3); 228-31
27. Li Z, Liu F, Cheng S, Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China: Sci Rep, 2016; 6; 21039
28. Gao L, Zhou F, Li X, Anal HPV infection in HIV-positive men who have sex with men from China: PLoS One, 2010; 5(12); e15256
29. Sammarco ML, Ucciferri C, Tamburro M, High prevalence of human papillomavirus type 58 in HIV infected men who have sex with men: A preliminary report in Central Italy: J Med Virol, 2016; 88(5); 911-14
30. Li X, Li M, Yang Y, Anal HPV/HIV co-infection among men who have sex with men: A cross-sectional survey from three cities in China: Sci Rep, 2016; 6(1); 1-9
31. Yin WG, Yang M, Peng L, Male papillomavirus infection and genotyping in the Qingyuan area: Virol J, 2020; 17(1); 1-7
32. He Z, Liu Y, Sun Y, Human papillomavirus genital infections among men, China, 2007–2009: Emerg Infect Dis, 2013; 19(6); 992
33. Skoulakis A, Fountas S, Mantzana-Peteinelli M, Prevalence of human papillomavirus and subtype distribution in male partners of women with cervical intraepithelial neoplasia (CIN): A systematic review: BMC Infect Dis, 2019; 19(1); 192
34. Sudenga SL, Torres BN, Silva R, Comparison of the natural history of genital HPV infection among men by country: Brazil, Mexico, and the United States: Cancer Epidem Biomar, 2017; 26(7); 1043-52
35. Matsuzawa Y, Kitamura T, Suzuki M, Prevalence, genotype distribution, and predictors against HPV infections targeted by 2-, 4-, 9-valent hpv vaccines among Japanese males: Vaccines, 2020; 8(2); 221
36. Wu DD, Long FQ, Gao J, HPV6 and HPV11 genome methylation in condyloma accuminatum measured by bisulfite sequencing: Am J Dermatopathol, 2019; 41(7); 534-35
37. Vonsky M, Shabaeva M, Runov A, Carcinogenesis associated with human papillomavirus infection: Mech Potential Immunother Biochem, 2019; 84(7); 782-99
38. Bao Y, Li N, Smith J, Qiao Y, Human papillomavirus type-distribution in the cervix of Chinese women: A meta-analysis: Int J STD AIDS, 2008; 19(2); 106-11
39. Luo G, Sun X, Li M, Cervical human papillomavirus among women in Guangdong, China 2008–2017: Implication for screening and vaccination: J Med Virol, 2019; 91(10); 1856-65
40. Qian L, Zhang Y, Cui D, Analysis of epidemiological trends in human papillomavirus infection among gynaecological outpatients in Hangzhou, China, 2011–2015: BMC Infect Dis, 2017; 17(1); 393
41. Fathi R, Tsoukas MM, Genital warts and other HPV infections: Established and novel therapies: Clin Dermatol, 2014; 32(2); 299-306
42. Park I0U, Introcaso C, Dunne EF, Human papillomavirus and genital warts: a review of the evidence for the 2015 Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines: Clin Infect Dis, 2015; 61(Suppl 8); S849-55
43. Kazlouskaya M, Fiadorchanka N, Regression of giant condyloma acuminata after one dose of 9-valent human papillomavirus (HPV) vaccine: Int J Dermatol, 2019; 58(12); e245-47
44. Lin W, Wang Y, Liu Z, Awareness and attitude towards human papillomavirus and its vaccine among females with and without daughter(s) who participated in cervical cancer screening in Shenzhen, China: Trop Med Int Health, 2019; 24(9); 1054-63
Figures
 Figure 1. Changes in the infection cases and infection rates of human papillomavirus (HPV) in male patients who visted our sexually transmitted disease (STD) clinic over 2015 to 2019.
Figure 1. Changes in the infection cases and infection rates of human papillomavirus (HPV) in male patients who visted our sexually transmitted disease (STD) clinic over 2015 to 2019. Figure 2. Type-specific prevalence of HPV among men in sexually transmitted disease (STD) clinic, 2015–2019. (A) LR-HPV types; (B) HR-HPV types. Vertical bars represent 95% confidence intervals.
Figure 2. Type-specific prevalence of HPV among men in sexually transmitted disease (STD) clinic, 2015–2019. (A) LR-HPV types; (B) HR-HPV types. Vertical bars represent 95% confidence intervals. Figure 3. Prevalence of HR, low-risk(LR) and any HPV infection in different age groups.
Figure 3. Prevalence of HR, low-risk(LR) and any HPV infection in different age groups. Figure 4. The detection rates among 9v HPV genotypes (A), non-9v HR-HPV genotypes (B) and non-9v LR-HPV genotypes (C).
Figure 4. The detection rates among 9v HPV genotypes (A), non-9v HR-HPV genotypes (B) and non-9v LR-HPV genotypes (C). Tables
 Table 1. Detection of HPV genotypes of male patients in a sexually transmitted disease clinic from 2015 to 2019.
Table 1. Detection of HPV genotypes of male patients in a sexually transmitted disease clinic from 2015 to 2019. Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019.
Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019. Table 3. Changes of type-specific genital human papillomavirus detection across different age groups.
Table 3. Changes of type-specific genital human papillomavirus detection across different age groups. Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019.
Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019. Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years.
Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years. Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types.
Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types. Table 1. Detection of HPV genotypes of male patients in a sexually transmitted disease clinic from 2015 to 2019.
Table 1. Detection of HPV genotypes of male patients in a sexually transmitted disease clinic from 2015 to 2019. Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019.
Table 2. Distribution of infection in patients from sexually transmitted disease clinic with 27 genotypes of human papillomavirus from 2015 to 2019. Table 3. Changes of type-specific genital human papillomavirus detection across different age groups.
Table 3. Changes of type-specific genital human papillomavirus detection across different age groups. Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019.
Table 4. Changes in the rate of male human papillomavirus infection in sexually transmitted disease clinic in different age groups from 2015 to 2019. Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years.
Table 5. Prevalence by human papillomavirus type in male patients by age groups over 5 years. Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types.
Table 6. Detection rates of human papillomavirus in male patients with different diagnosis types. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








