11 May 2021: Original Paper
Surgical Outcome of Pulmonary Metastasectomy for Hepatocellular Carcinoma Recurrence in Liver Transplant Patients
Yong Ho Jeong1ABCDEFG, Shin Hwang2ACDEF, Geun Dong Lee1AB, Se Hoon Choi1AB, Hyeong Ryul Kim1AB, Yong-Hee Kim1AB, Seung-Il Park1AB, Dong Kwan Kim1ABCDEFG*DOI: 10.12659/AOT.930383
Ann Transplant 2021; 26:e930383
Abstract
BACKGROUND: Recurrence of hepatocellular carcinoma (HCC) after liver transplantation (LT) results in poor survival outcome. This study assessed the clinical outcomes of pulmonary metastasectomy in LT recipients with pulmonary metastasis of HCC in a high-volume transplant center and analyzed factors prognostic of survival following metastasectomy.
MATERIAL AND METHODS: This study analyzed outcomes in 52 patients who underwent pulmonary resection due to pulmonary metastasis as the first recurrence of HCC after LT from January 2004 to December 2017 in a single center.
RESULTS: The 52 enrolled patients included 46 men and 6 women, aged 56.0±6.6 years. Their 1-, 3-, and 5-year survival rates after pulmonary resection were 75.0%, 43.5%, and 33.9%, respectively. The 1-, 3-, and 5-year survival rates were 85.3%, 47.1%, and 34.2%, respectively, in patients with further metastases and 55.6%, 38.1%, and 38.1%, respectively, in patients without further metastases (P=0.45). The size and number of pulmonary metastatic nodules were unrelated to survival rates (all P>0.10). A shorter recurrence-free period after LT (hazard ratio [HR]=0.553, P=0.006), elevated alpha-fetoprotein concentration at metastasectomy (HR=2.142, P=0.03), and adjuvant chemotherapy after metastasectomy (HR=3.79, P=0.003) were independent risk factors for survival after metastasectomy.
CONCLUSIONS: Pulmonary metastasectomy for HCC recurrence in LT recipients showed favorable survival outcomes. Independent risk factors for survival after metastasectomy included recurrence-free survival after LT, alpha-fetoprotein level at metastasectomy, and adjuvant chemotherapy after metastasectomy.
Keywords: Carcinoma, Hepatocellular, Liver Transplantation, metastasectomy, Liver Neoplasms, Pneumonectomy
Background
Liver transplantation (LT) is considered an established treatment for hepatocellular carcinoma (HCC), which has resulted in the increased performance of LT in patients with HCC [1,2]. Although candidates for LT are carefully selected, HCC recurrence remains the main cause of patient death [1]. The rates of HCC recurrence after LT vary from 7% to 40% and are generally about 20% [3–5]. Most extrahepatic metastases after LT occur in the lungs [6]. Pulmonary metastasectomy is considered the preferred treatment for pulmonary metastases, following liver resection or LT for HCC [7–12]. By contrast, chemotherapy for patients with post-transplant pulmonary metastasis has shown low treatment response rates, with 1-year survival rates between 0% and 30% [13]. To determine the efficacy of pulmonary metastasectomy for patients with pulmonary metastases following LT for HCC, this study assessed the clinical outcomes in LT recipients who underwent pulmonary metastasectomy at a high-volume transplant center and analyzed factors prognostic of post-metastasectomy patient survival.
Material and Methods
PATIENT SELECTION:
This single-center, retrospective observational study included patients who underwent LT for HCC at Asan Medical Center during the 14-year study period between January 2004 and December 2017 and later underwent resection of post-transplant pulmonary metastases. The study patients were followed up until December 2019 or patient death through review of institutional medical records and with the assistance of the National Health Insurance Service in Korea. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-1298), which waived the requirement for informed consent owing to the retrospective design of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
PATIENT GROUPING:
Patients were grouped by recurrence-free period after LT (≤1 year, >1 but ≤2 years, or >2 years), recurrence after pulmonary metastasectomy (yes or no), number of pulmonary metastases (1, 2, or ≤3), size of the largest metastatic nodule (≤10 mm, >10 but ≤20 mm, and >20 mm), administration of postoperative chemotherapy (yes or no), and alpha-fetoprotein (AFP) concentration at the time of metastasectomy (≤10 ng/mL or >10 ng/mL).
INDICATIONS AND SURGICAL TECHNIQUES FOR PULMONARY METASTASECTOMY:
The surgical indications for pulmonary metastasectomy for post-transplant HCC recurrence included a general operable condition, location of the pulmonary metastasis in a resectable portion of the lung, controlled HCC at the primary site, and no other metastases detected in other organs. Patients who had previous intrahepatic or extrahepatic metastases before pulmonary metastasis were excluded.
Wedge resection of the pulmonary metastatic lesion was the surgical treatment of choice. Lobectomy was performed when the nodules were centrally located, precluding wedge resection for total resection of the metastatic lesion. Most patients in this study underwent video-assisted thoracoscopic surgery. Factors associated with the metastasectomy were recorded, including the duration of hospital stay and postoperative complications.
POSTOPERATIVE FOLLOW-UP:
All patients who underwent LT at the Asan Medical Center were assessed by follow-up chest computed tomography (CT) 3 months after transplantation. Patients within the Milan criteria at the time of LT underwent subsequent chest CT every 6 months up to 3 years, every year up to 5 years, and every 2 years thereafter. Patients who were beyond the Milan criteria at LT underwent subsequent CT every 3 months for 1 year, every 4 to 6 months up to 3 years, every year up to 5 years, and every 2 years thereafter.
STATISTICAL ANALYSIS:
Numerical data are presented as mean±standard deviation (SD) or median with range. Overall survival and recurrence-free survival rates were estimated using the Kaplan-Meier method and compared with the log-rank test. The Cox regression model was used for multivariate analysis. SPSS version 21 (IBM, New York, NY, USA) was used for statistical analyses.
Results
PATIENT PROFILES:
A total of 4699 LTs were performed during the 14-year study period from January 2004 to December 2017. HCC was diagnosed in 2230 LT recipients, with HCC recurring in 283 patients, of which 77 patients underwent pulmonary metastasectomy for post-transplant HCC recurrence. Because 25 of these patients had recurrences at other sites prior to a pulmonary recurrence, and therefore pulmonary metastasis was not the initial tumor recurrence, these patients were excluded. Thus, 52 patients were included in this study. Their clinicopathologic profiles are shown in Table 1. Of these 52 patients, 28 (53.8%) experienced recurrence within 1 year, 7 (13.5%) experienced recurrence between 1 and 2 years, and 17 (32.7%) experienced recurrence after 2 years.
Of the 52 patients, 50 (96.2%) underwent wedge resection and 2 (3.8%) underwent lobectomy. Video-assisted thoracoscopic surgery was performed in 47 (90.4%) patients and open surgery was performed in 5 (9.6%). The median hospital stay after metastasectomy was 3 days (range, 2–22 days). Persistent air leak lasting longer than 7 days was the only surgical complication, occurring in 4 (7.7%) patients. There was no postoperative patient mortality.
Of the 52 patients, 33 were administered adjuvant chemotherapy following pulmonary metastasectomy, with 28 of these patients receiving sorafenib. There are no clear guidelines regarding the administration of adjuvant chemotherapy after pulmonary metastasectomy in our institution.
OVERALL SURVIVAL AND FURTHER RECURRENCE:
The overall mean±SD follow-up period after LT was 5.4±4.1 years (range, ~0.2–14.2 years; median, 4.0 years). Patient survival and recurrence data as of December 2019 are shown in Table 2. Of the 52 patients, 34 (65.4%) experienced further recurrence after pulmonary metastasectomy. The mean ± SD recurrence-free period after metastasectomy was 1.7±2.4 years (range, ~0.1–12.0 years; median, 0.6 years) (Table 2). The most common site of further recurrence was the lungs, occurring in 25 (48.1%) patients. Around 30% of these patients had multiple recurrences after metastasectomy. Of the 25 patients with further lung recurrence, 20 underwent repeat metastasectomy. Patients with repeated pulmonary metastases that were non-operable were administered chemotherapy or stereotactic body radiation therapy. Recurrences in the liver were preferentially treated with transcatheter arterial chemoembolization. Metastases at other sites were treated locally, if possible; for example, bone metastases were treated with radiotherapy and brain metastases with the gamma knife.
The overall 1-, 3-, and 5-year patient survival rates were 90.4%, 65.3%, and 46.8%, respectively, after LT (Figure 1A), and 75.0%, 43.5%, and 33.9%, respectively, after metastasectomy (Figure 1B). The 1-, 3-, and 5-year recurrence-free survival rates after metastasectomy were 52.2%, 29.2%, and 19.5%, respectively (Figure 2).
Ten patients died without further HCC recurrence (Table 2). Patient survival status was assessed with the assistance of the National Health Insurance Service in Korea; thus, the detailed causes of patient death were not clarified unless the patients regularly visited our institution. One patient each died due to intracranial hemorrhage and pneumonia.
EFFECTS OF RECURRENCE-FREE PERIOD AND FURTHER RECURRENCE ON POST-METASTASECTOMY SURVIVAL:
The 1-, 3-, and 5-year post-metastasectomy survival rates were 64.3%, 31.4%, and 26.9%, respectively, in patients who experienced recurrence within 1 year; 85.7%, 57.1%, and 57.1%, respectively, in patients who experienced recurrence at 1 to 2 years; and 88.2%, 57.8%, and 57.8%, respectively, in patients who experienced recurrence after 2 years (Figure 3A). The difference among these groups was statistically significant (P=0.014), with a longer recurrence-free period showing better prognosis.
The 1-, 3-, and 5-year post-metastasectomy survival rates were 85.3%, 47.1%, and 34.2%, respectively, in patients with further metastasis after metastasectomy and 55.6%, 38.1%, and 38.1%, respectively, in patients without further recurrence (P=0.45) (Figure 3B).
EFFECTS OF LESION SIZE AND NUMBER ON POST-METASTASECTOMY SURVIVAL:
The median number of pulmonary metastasis lesion was 1 (range, 1–7). The 1-, 3-, and 5-year post-metastasectomy survival rates were 82.4%, 43.8%, and 29.6%, respectively, in patients with a single metastatic site; 72.7%, 54.5%, and 54.5%, respectively, in patients with 2 metastases; and 42.9%, 28.6%, and 28.6%, respectively, in patients with more than 2 metastases. The differences were not statistically significant (P=0.43) (Figure 4A).
The median size of the largest pulmonary metastasis was 1.2 cm (range, 0.2–3.5 cm). The 1-, 3-, and 5-year post-metastasectomy survival rates were 76.2%, 61.5%, and 43.8%, respectively, in patients with lesions ≤10 mm; 80.0%, 28.0%, and 22.4%, respectively, in patients with lesions >10 mm but ≤20 mm; and 50.0%, 50.0%, and 50.0%, respectively, in patients with lesions >20 mm. The differences were not statistically significant (P=0.41) (Figure 4B).
EFFECTS OF AFP LEVEL AT PULMONARY METASTASECTOMY ON POST-METASTASECTOMY SURVIVAL:
The median serum concentration of alpha-fetoprotein (AFP) at the time of pulmonary metastasectomy in these 52 patients was 3.9 ng/mL (range, 0.7–4769.9 ng/mL). Of these 52 patients, 34 (65.4%) had normal serum AFP concentrations and 18 (34.6%) had elevated serum AFP concentrations at the time of pulmonary metastasectomy. The 1-, 3-, and 5-year post-metastasectomy survival rates were 85.3%, 54.7%, and 39.1%, respectively, in patients with normal AFP; and 55.6%, 22.2%, and 22.2%, respectively, in patients with elevated AFP (Figure 5), indicating that patients with normal AFP levels survived significantly longer than those with elevated AFP (P=0.027).
EFFECTS OF POST-METASTASECTOMY CHEMOTHERAPY ON POST-METASTASECTOMY SURVIVAL:
The 1-, 3-, and 5-year post-metastasectomy survival rates were 78.9%, 68.4%, and 62.7%, respectively, in patients who did not receive chemotherapy and 72.7%, 27.8%, and 12.3%, respectively, in patients who received chemotherapy (Figure 6), indicating that patients who did not receive post-metastasectomy chemotherapy survived significantly longer than those who did receive post-metastasectomy chemotherapy (P=0.002).
FACTORS PROGNOSTIC OF POST-METASTASECTOMY SURVIVAL:
Univariate analyses showed that recurrence-free period before metastasectomy (P=0.014), AFP concentration at the time of pulmonary metastasectomy (P=0.027), and postoperative chemotherapy (P=0.002) were factors predictive of post-metastasectomy patient survival (Table 3). Multivariate analysis showed that all 3 factors were independently predictive of post-metastasectomy patient survival: recurrence-free period (hazard ratio [HR] 0.56, 95% confidence interval [CI] 0.37–0.86; P=0.008), AFP concentration (HR 2.79, 95% CI 1.36–5.72; P=0.005), and postoperative chemotherapy (HR 3.68, 95% CI 1.49–9.07; P=0.005) (Table 3).
Discussion
In addition to liver resection, LT has become a definitive treatment for HCC, with studies reporting better long-term overall survival and disease-free survival with LT than with liver resection [14]. Moreover, criteria for LT have expanded [15]. Extrahepatic recurrence after LT is the most frequent cause of patient mortality, with the lungs being one of the most common sites of extrahepatic metastases [16]. For example, of 439 HCC patients visiting a single center for treatment, 50 had pulmonary metastases [17], and a retrospective study in 403 HCC patients visiting a single center for treatment identified 148 extrahepatic metastases, including 80 pulmonary metastases, suggesting that pulmonary metastases are the most common type of extrahepatic HCC metastases [18]. The present study also found that the lungs were the most common site of extrahepatic metastases after LT.
LT recipients differ from patients who undergo hepatic resection, as LT recipients have a lower degree of liver cirrhosis and must use immunosuppressants for life. Few studies to date have assessed outcomes of pulmonary metastasectomy for recurrent HCC in patients after LT, and those studies have involved relatively short-term follow-up results in relatively few patients [8,16]. For example, a study of 104 patients who underwent LT for HCC, including 5 with pulmonary recurrence, showed that survival times were comparable for pulmonary metastasectomy and liver resection [8]. Similarly, a study of 43 patients who underwent LT for HCC and later developed pulmonary metastases, including 23 who underwent pulmonary metastasectomy and 20 who did not, found that the former had a better prognosis [16]. To our knowledge, the present study is the largest to date to assess outcomes in patients who underwent pulmonary metastasectomy for HCC following LT.
Organ transplantation is considered a risk factor for the development of malignancy. The International Society for Heart and Lung Transplantation has reported that about 25% of these patients experienced a new type of malignancy within 5 years after transplantation, increasing to 50% of patients within 12 years [19]. Immunosuppressants administered to these patients after transplantation can promote cancer cell proliferation [20,21]. Although several studies have tested the effects of different immunosuppressants on tumor recurrence after organ transplantation [22–24], further studies are needed.
Pulmonary metastasectomy of HCC recurrence in patients without LT has also shown effectiveness, with 5-year survival rates between 11.8% and 66.9% [10–12]. The present study showed that pulmonary metastasectomy in LT recipients yielded favorable results with a 5-year survival rate after metastasectomy of 33.9%, which is similar to that in non-LT patients. For example, the 1- and 3-year recurrence-free survival rates following pulmonary metastasectomy in patients who underwent liver resection for HCC were 32.4% and 21.6%, respectively [25]. In addition, a study of pulmonary metastasectomy in 19 patients, including 18 who underwent liver resection and 1 who underwent transarterial chemoembolization for HCC, reported 1-, 3-, and 5-year recurrence-free survival rates of 47%, 35%, and 28%, respectively [26]. In the present study, the 1-, 3-, and 5-year recurrence-free survival rates after pulmonary metastasectomy were 59.2%, 29.2%, and 19.5%, respectively, suggesting that pulmonary metastasectomy yields similar results in HCC patients following LT or hepatic resection. Hwang et al reported the results of 23 LT-receiving patients who received pulmonary metastasectomy after HCC recurrence with a post-metastasectomy 3-year survival rate of 30.6% [16]. This present study included a higher number of patients and showed an improved survival rate at 5 years after metastasectomy.
Most patients with pulmonary metastasis have no symptoms, and therefore screening according to patients’ symptoms will likely miss recurrence until a later stage. In the present study, 53.8% of patients experienced pulmonary recurrence within 1 year after LT, with the median recurrence-free period between LT and pulmonary metastasis being 0.9 years. Similarly, a study of 93 patients who underwent pulmonary metastasectomy following hepatic resection for HCC showed a median recurrence-free period of 17 months [10]. Close follow-up after LT for HCC, especially by chest CT, is required to detect early recurrence, resulting in more rapid treatment and better survival outcomes.
The present study found that early recurrence of HCC, elevated AFP level at the time of pulmonary metastasectomy, and administration of adjuvant chemotherapy were prognostic of overall patient survival. Similarly, the disease-free interval was reported to be an independent prognostic factor for survival in patients who underwent pulmonary metastasectomy for HCC recurrence [10]. Measuring blood AFP level is an easy way to follow up with HCC patients after LT. Other studies have also reported that elevated AFP is prognostic of recurrence [27,28]. Moreover, AFP was found to promote metastasis of HCC, both in vitro and in vivo [29]. Little is known about the association between AFP and survival in patients undergoing LT. Although our study found that elevated AFP level was prognostic of poor survival, it is unclear whether AFP directly affects survival or whether survival is reduced due to early recurrence. Hwang et al were unable to find prognostic factors in 2012 [16], but the present study was able to identify these statistically significant prognostic factors.
Our present finding, that post-metastasectomy adjuvant chemotherapy was an independent risk factor for survival, was primarily owing to patient selection bias. Patients were selected for adjuvant chemotherapy plus metastasectomy because they had multiple and/or large metastatic lesions, putting them at greater risk of post-metastasectomy recurrence. Administration of chemotherapy to patients with HCC recurrence, with or without locoregional therapy, has been found to result in poor outcomes [30,31]. A study of 108 patients with HCC who received hepatic resection and adjuvant chemotherapy found that long-term outcomes were poor and recurrence rates were increased [32]. Administration of adjuvant metformin to 304 patients who underwent hepatic resection and 74 who underwent LT found no antitumor effect [31]. The randomized, double-blind, placebo-controlled, phase 3 STORM trial, which included over 1100 patients with HCC who underwent hepatic resection, found that, after a median duration of 12.5 months, there was no difference in recurrence-free survival or overall survival between groups who were either treated or not treated with sorafenib [33]. Our results showed that administration of chemotherapy was associated with poor prognosis after pulmonary metastasectomy.
This study had several limitations, including its retrospective design and the inclusion of relatively few patients from a single center. Inclusion of patients who underwent metastasectomy was highly selective, in that unresectable patients were excluded. There are no clear protocols for postoperative adjuvant chemotherapy, which could have affected survival. Further studies with larger numbers of patients or multicenter trials are required.
Conclusions
Pulmonary metastasectomy in selected patients with pulmonary metastasis following LT for HCC may enhance long-term survival. Early post-transplant recurrence, elevated AFP level, and adjuvant chemotherapy after metastasectomy were significantly prognostic for patient survival after metastasectomy.
Figures
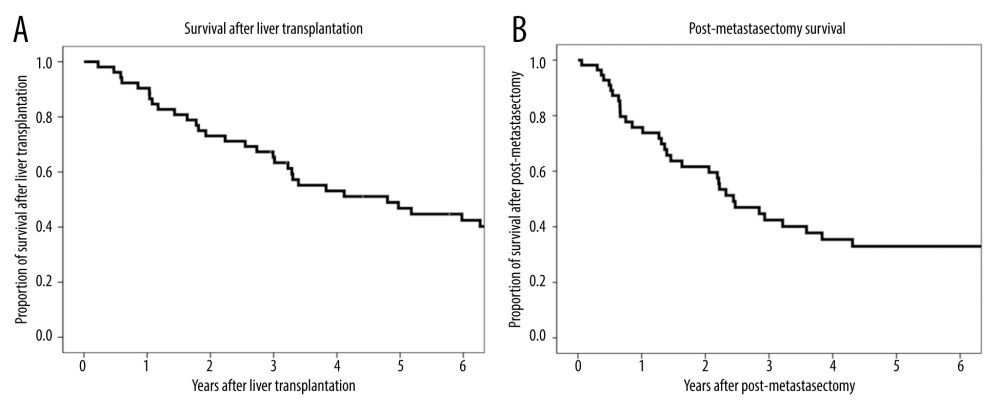 Figure 1. (A) Kaplan-Meier analysis of overall patient survival after liver transplantation. (B) Kaplan-Meier analysis of overall patient survival after metastasectomy.
Figure 1. (A) Kaplan-Meier analysis of overall patient survival after liver transplantation. (B) Kaplan-Meier analysis of overall patient survival after metastasectomy. 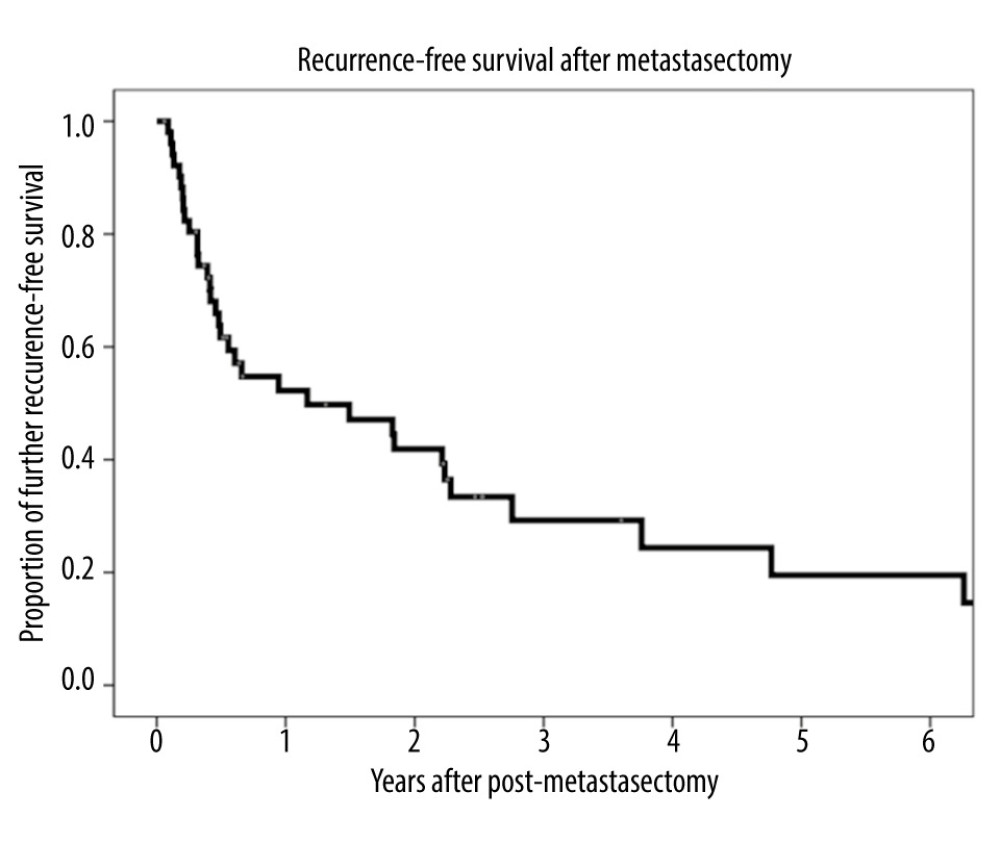 Figure 2. Kaplan-Meier analysis of recurrence-free survival after metastasectomy.
Figure 2. Kaplan-Meier analysis of recurrence-free survival after metastasectomy. 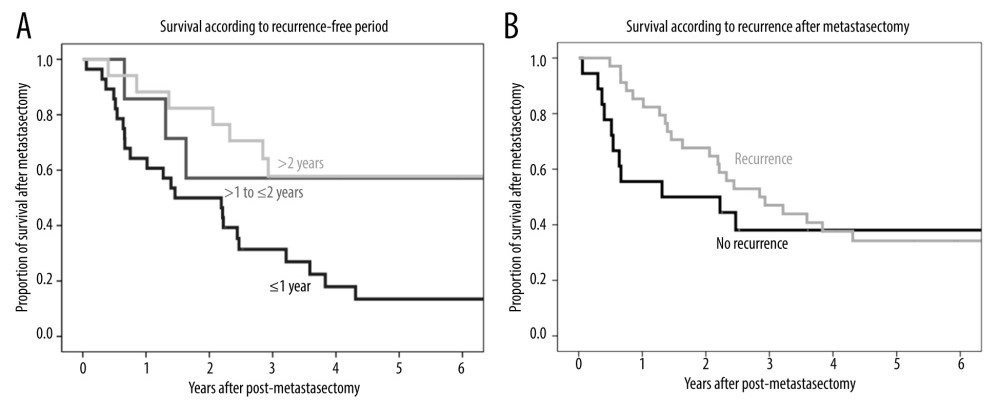 Figure 3. (A) Kaplan-Meier analysis of overall patient survival according to recurrence-free period (≤1 year, >1 to ≤2 years, >2 years) after metastasectomy. (B) Kaplan-Meier analysis of overall patient survival in patients with and without additional recurrence after metastasectomy.
Figure 3. (A) Kaplan-Meier analysis of overall patient survival according to recurrence-free period (≤1 year, >1 to ≤2 years, >2 years) after metastasectomy. (B) Kaplan-Meier analysis of overall patient survival in patients with and without additional recurrence after metastasectomy. 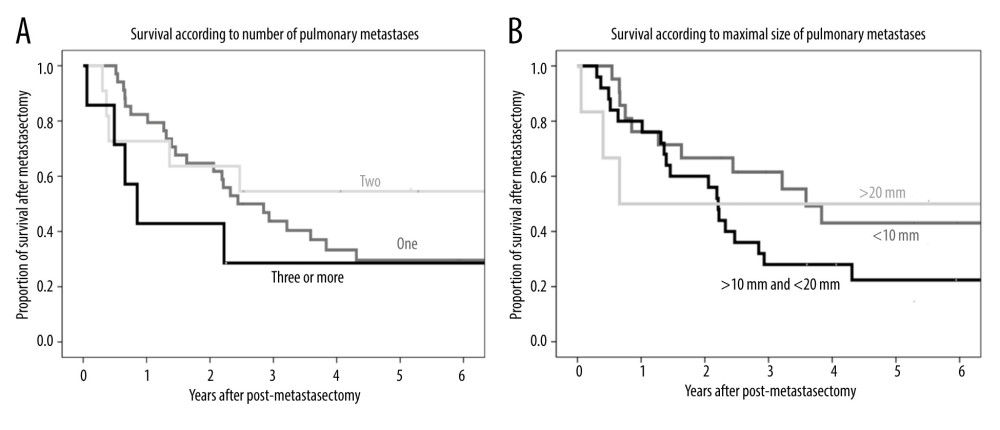 Figure 4. (A) Kaplan-Meier analysis of patient survival according to number of pulmonary metastases (1, 2, or ≥3). (B) Kaplan-Meier analysis of patient survival according to maximal size of pulmonary metastases (≤10 mm, >10 to ≤20 mm, or >20 mm).
Figure 4. (A) Kaplan-Meier analysis of patient survival according to number of pulmonary metastases (1, 2, or ≥3). (B) Kaplan-Meier analysis of patient survival according to maximal size of pulmonary metastases (≤10 mm, >10 to ≤20 mm, or >20 mm). 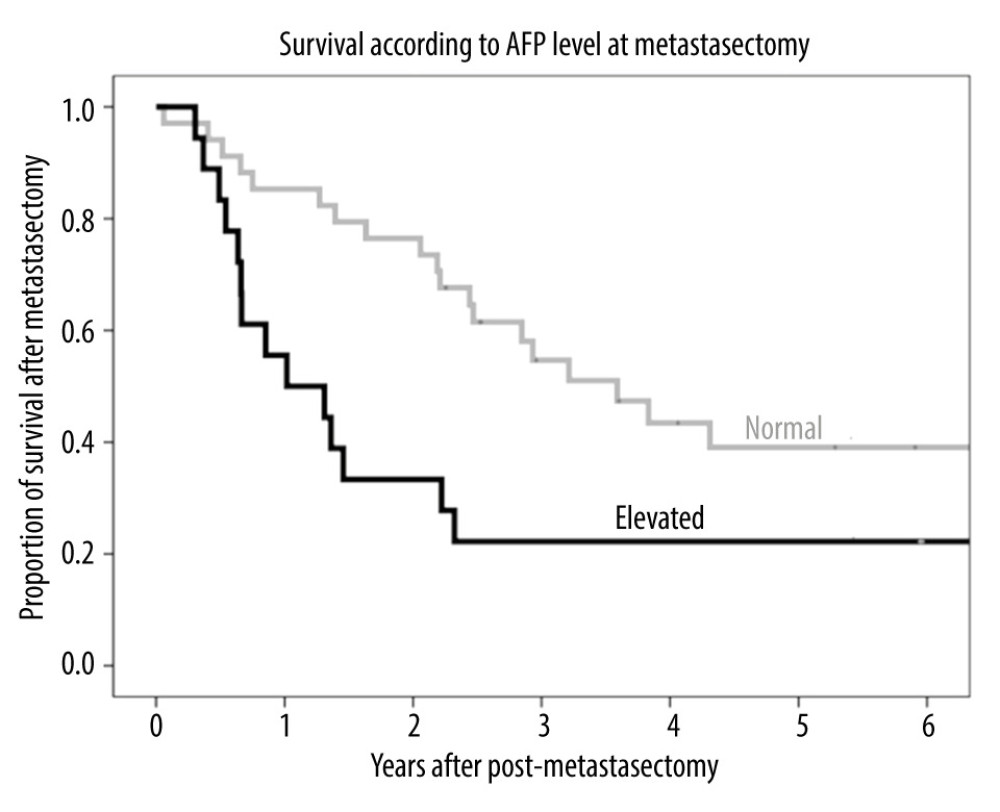 Figure 5. Kaplan-Meier analysis of patient survival according to AFP level (normal or elevated) before metastasectomy.
Figure 5. Kaplan-Meier analysis of patient survival according to AFP level (normal or elevated) before metastasectomy. 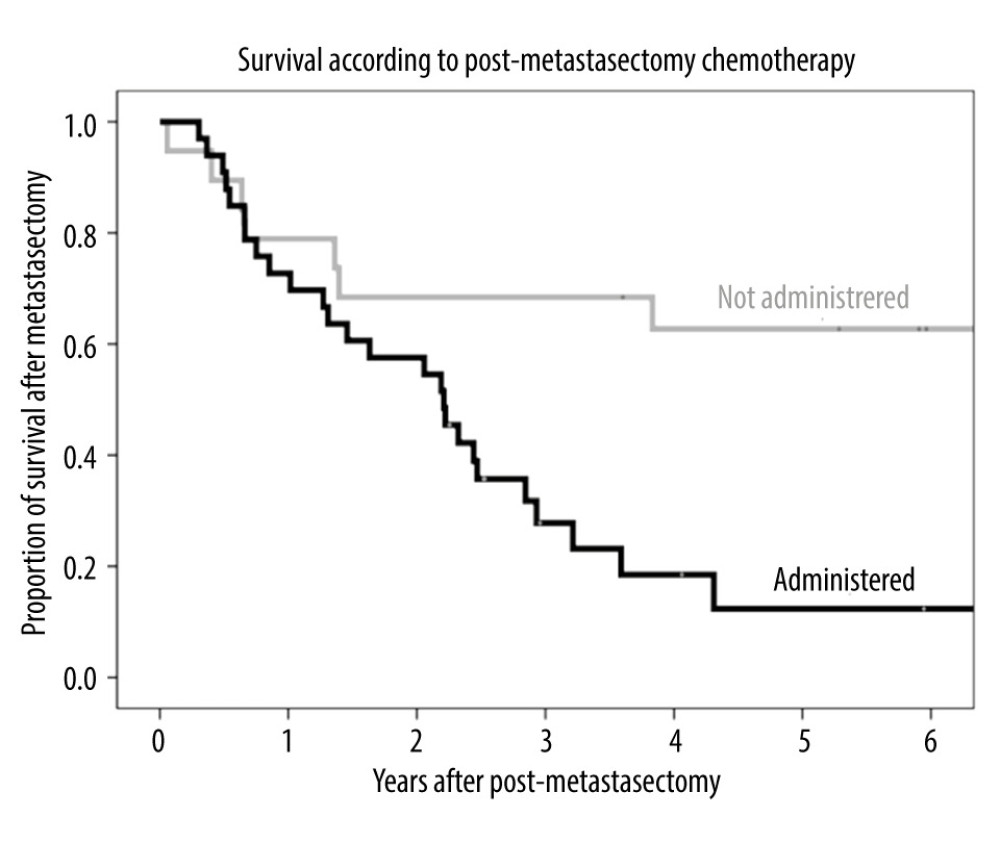 Figure 6. Kaplan-Meier analysis of overall survival in patients who received chemotherapy and did not receive chemotherapy after metastasectomy.
Figure 6. Kaplan-Meier analysis of overall survival in patients who received chemotherapy and did not receive chemotherapy after metastasectomy. Tables
Table 1. Clinicopathological characteristics of the 52 patients who underwent pulmonary metastasectomy for lung metastases after liver transplantation for hepatocellular carcinoma.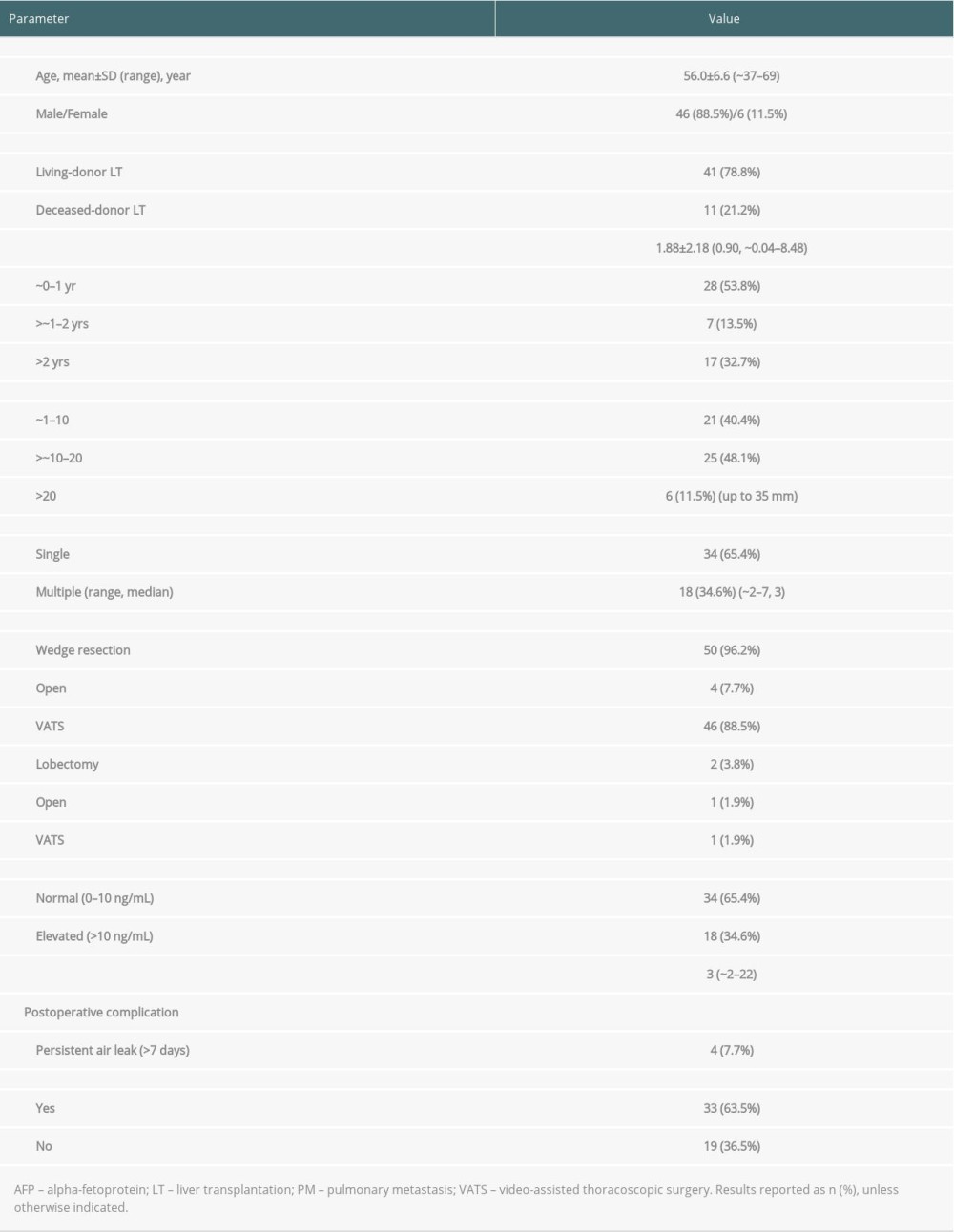 Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy.
Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy.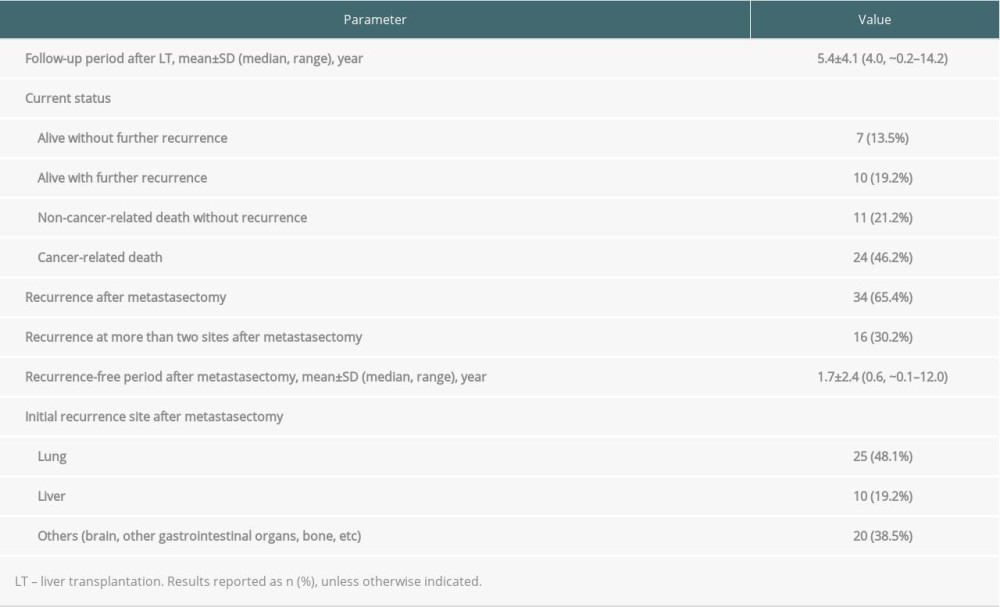 Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival.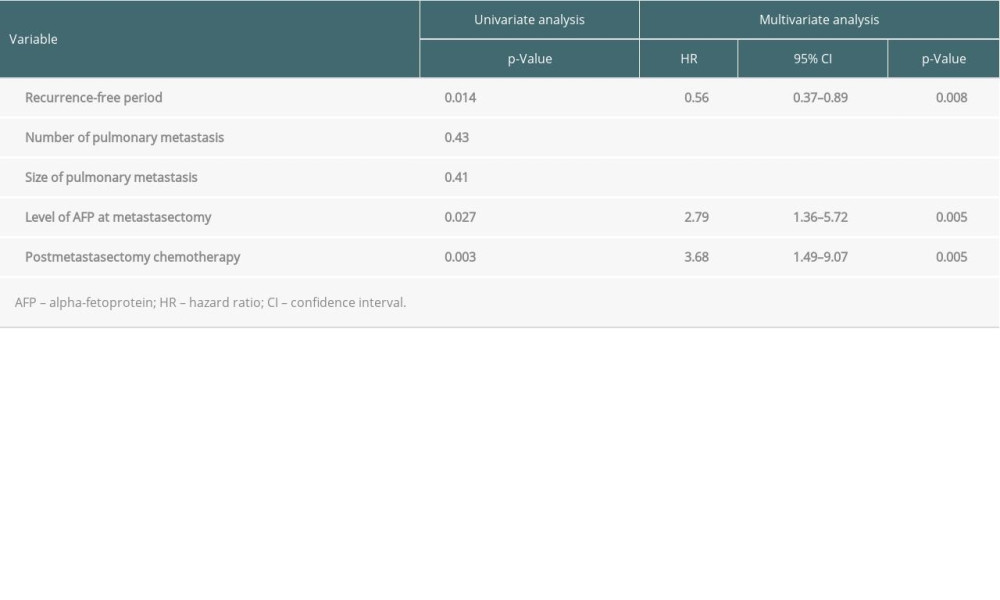
References
1. Lee SG, Hwang S, Moon DB, Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center: Liver Transpl, 2008; 14(7); 935-45
2. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334(11); 693-99
3. Zimmerman MA, Ghobrial RM, Tong MJ, Recurrence of hepatocellular carcinoma following liver transplantation: A review of preoperative and postoperative prognostic indicators: Arch Surg, 2008; 143(2); 182-88 discussion 188
4. Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ, The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time: J Clin Oncol, 2003; 21(23); 4329-35
5. Marsh JW, Dvorchik I, Subotin M, The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: A pilot study: Hepatology, 1997; 26(2); 444-50
6. Uka K, Aikata H, Takaki S, Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma: World J Gastroenterol, 2007; 13(3); 414-20
7. Cho S, Ryu KM, Hwang YJ, Lee EB, Prognostic factors for pulmonary metastasectomy in the treatment of hepatocellular carcinoma: J Thorac Oncol, 2010; 5(8); 1251-54
8. Bates MJ, Farkas E, Taylor D, McFadden PM, Pulmonary resection of metastatic hepatocellular carcinoma after liver transplantation: Ann Thorac Surg, 2008; 85(2); 412-15
9. Nakagawa T, Kamiyama T, Nakanishi K, Pulmonary resection for metastases from hepatocellular carcinoma: Factors influencing prognosis: J Thorac Cardiovasc Surg, 2006; 131(6); 1248-54
10. Takahashi Y, Ikeda N, Nakajima J, Prognostic analysis of surgical resection for pulmonary metastasis from hepatocellular carcinoma: World J Surg, 2016; 40(9); 2178-85
11. Han KN, Kim YT, Yoon JH, Role of surgical resection for pulmonary metastasis of hepatocellular carcinoma: Lung Cancer, 2010; 70(3); 295-300
12. Yoon YS, Kim HK, Kim J, Long-term survival and prognostic factors after pulmonary metastasectomy in hepatocellular carcinoma: Ann Surg Oncol, 2010; 17(10); 2795-801
13. Pokorny H, Gnant M, Rasoul-Rockenschaub S, Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation?: Am J Transplant, 2005; 5(4 Pt 1); 788-94
14. Xu XS, Liu C, Qu K, Song YZ, Liver transplantation versus liver resection for hepatocellular carcinoma: A meta-analysis: Hepatobiliary Pancreat Dis Int, 2014; 13(3); 234-41
15. Lee HW, Suh KS, Advancements of liver transplantation for hepatocellular carcinoma in Korea: Jpn J Clin Oncol, 2017; 47(2); 93-100
16. Hwang S, Kim Y-H, Kim DK, Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation: World J Surg, 2012; 36(7); 1592-602
17. Tsai GL, Liu JD, Siauw CP, Chen PH, Thoracic roentgenologic manifestations in primary carcinoma of the liver: Chest, 1984; 86(3); 430-34
18. Katyal S, Oliver JH, Peterson MS, Extrahepatic metastases of hepatocellular carcinoma: Radiology, 2000; 216(3); 698-703
19. Chambers DC, Cherikh WS, Harhay MO, The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation report-2019; Focus theme: Donor and recipient size match: J Heart Lung Transplant, 2019; 38(10); 1042-55
20. Hojo M, Morimoto T, Maluccio M, Cyclosporine induces cancer progression by a cell-autonomous mechanism: Nature, 1999; 397(6719); 530-34
21. Maluccio M, Sharma V, Lagman M, Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression: Transplantation, 2003; 76(3); 597-602
22. Semela D, Piguet AC, Kolev M, Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma: J Hepatol, 2007; 46(5); 840-48
23. Grigg SE, Sarri GL, Gow PJ, Yeomans ND, Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma: Aliment Pharmacol Ther, 2019; 49(10); 1260-73
24. Geissler EK, Schnitzbauer AA, Zülke C, Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomized, multicenter, open-label phase 3 trial: Transplantation, 2016; 100(1); 116-25
25. Nakajima J, Tanaka M, Matsumoto J, Appraisal of surgical treatment for pulmonary metastasis from hepatocellular carcinoma: World J Surg, 2005; 29(6); 715-18
26. Mizuguchi S, Nishiyama N, Izumi N, Clinical significance of multiple pulmonary metastasectomy for hepatocellular carcinoma: World J Surg, 2016; 40(2); 380-87
27. Huang LF, Wan P, Xu DW, Nomogram predicting pulmonary metastasis of hepatocellular carcinoma after liver transplantation: Oncotarget, 2018; 9(2); 2425-34
28. Jung SM, Kim JM, Choi GS, Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma: J Gastrointest Surg, 2019; 23(2); 304-11
29. Lu Y, Zhu M, Li W, Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells: J Cell Mol Med, 2016; 20(3); 549-58
30. Akateh C, Black SM, Conteh L, Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma: World J Gastroenterol, 2019; 25(28); 3704-21
31. Chung YK, Hwang S, Song GW, Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation: Ann Hepatobiliary Pancreat Surg, 2018; 22(4); 297-304
32. Ono T, Yamanoi A, Nazmy El Assal O, Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: Metaanalysis of three randomized controlled trials: Cancer, 2001; 91(12); 2378-85
33. Bruix J, Takayama T, Mazzaferro V, Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial: Lancet Oncol, 2015; 16(13); 1344-54
Figures
 Figure 1. (A) Kaplan-Meier analysis of overall patient survival after liver transplantation. (B) Kaplan-Meier analysis of overall patient survival after metastasectomy.
Figure 1. (A) Kaplan-Meier analysis of overall patient survival after liver transplantation. (B) Kaplan-Meier analysis of overall patient survival after metastasectomy. Figure 2. Kaplan-Meier analysis of recurrence-free survival after metastasectomy.
Figure 2. Kaplan-Meier analysis of recurrence-free survival after metastasectomy. Figure 3. (A) Kaplan-Meier analysis of overall patient survival according to recurrence-free period (≤1 year, >1 to ≤2 years, >2 years) after metastasectomy. (B) Kaplan-Meier analysis of overall patient survival in patients with and without additional recurrence after metastasectomy.
Figure 3. (A) Kaplan-Meier analysis of overall patient survival according to recurrence-free period (≤1 year, >1 to ≤2 years, >2 years) after metastasectomy. (B) Kaplan-Meier analysis of overall patient survival in patients with and without additional recurrence after metastasectomy. Figure 4. (A) Kaplan-Meier analysis of patient survival according to number of pulmonary metastases (1, 2, or ≥3). (B) Kaplan-Meier analysis of patient survival according to maximal size of pulmonary metastases (≤10 mm, >10 to ≤20 mm, or >20 mm).
Figure 4. (A) Kaplan-Meier analysis of patient survival according to number of pulmonary metastases (1, 2, or ≥3). (B) Kaplan-Meier analysis of patient survival according to maximal size of pulmonary metastases (≤10 mm, >10 to ≤20 mm, or >20 mm). Figure 5. Kaplan-Meier analysis of patient survival according to AFP level (normal or elevated) before metastasectomy.
Figure 5. Kaplan-Meier analysis of patient survival according to AFP level (normal or elevated) before metastasectomy. Figure 6. Kaplan-Meier analysis of overall survival in patients who received chemotherapy and did not receive chemotherapy after metastasectomy.
Figure 6. Kaplan-Meier analysis of overall survival in patients who received chemotherapy and did not receive chemotherapy after metastasectomy. Tables
 Table 1. Clinicopathological characteristics of the 52 patients who underwent pulmonary metastasectomy for lung metastases after liver transplantation for hepatocellular carcinoma.
Table 1. Clinicopathological characteristics of the 52 patients who underwent pulmonary metastasectomy for lung metastases after liver transplantation for hepatocellular carcinoma. Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy.
Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy. Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival. Table 1. Clinicopathological characteristics of the 52 patients who underwent pulmonary metastasectomy for lung metastases after liver transplantation for hepatocellular carcinoma.
Table 1. Clinicopathological characteristics of the 52 patients who underwent pulmonary metastasectomy for lung metastases after liver transplantation for hepatocellular carcinoma. Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy.
Table 2. Recurrence and survival status of patients who underwent pulmonary metastasectomy. Table 3. Univariate and multivariate analysis of factors associated with patient survival.
Table 3. Univariate and multivariate analysis of factors associated with patient survival. In Press
15 Mar 2024 : Review article
Approaches and Challenges in the Current Management of Cytomegalovirus in Transplant Recipients: Highlighti...Ann Transplant In Press; DOI: 10.12659/AOT.941185
18 Mar 2024 : Original article
Does Antibiotic Use Increase the Risk of Post-Transplantation Diabetes Mellitus? A Retrospective Study of R...Ann Transplant In Press; DOI: 10.12659/AOT.943282
20 Mar 2024 : Original article
Transplant Nephrectomy: A Comparative Study of Timing and Techniques in a Single InstitutionAnn Transplant In Press; DOI: 10.12659/AOT.942252
28 Mar 2024 : Original article
Association Between FEV₁ Decline Rate and Mortality in Long-Term Follow-Up of a 21-Patient Pilot Clinical T...Ann Transplant In Press; DOI: 10.12659/AOT.942823
Most Viewed Current Articles
05 Apr 2022 : Original article
Impact of Statins on Hepatocellular Carcinoma Recurrence After Living-Donor Liver TransplantationDOI :10.12659/AOT.935604
Ann Transplant 2022; 27:e935604
12 Jan 2022 : Original article
Risk Factors for Developing BK Virus-Associated Nephropathy: A Single-Center Retrospective Cohort Study of ...DOI :10.12659/AOT.934738
Ann Transplant 2022; 27:e934738
22 Nov 2022 : Original article
Long-Term Effects of Everolimus-Facilitated Tacrolimus Reduction in Living-Donor Liver Transplant Recipient...DOI :10.12659/AOT.937988
Ann Transplant 2022; 27:e937988
15 Mar 2022 : Case report
Combined Liver, Pancreas-Duodenum, and Kidney Transplantation for Patients with Hepatitis B Cirrhosis, Urem...DOI :10.12659/AOT.935860
Ann Transplant 2022; 27:e935860








