Abstract
Background
High-risk lesions (HRL) of the breast are risk factors for future breast cancer development and may be associated with a concurrent underlying malignancy when identified on needle biopsy; however, there are few data evaluating HRLs in carriers of germline pathogenic variants (PVs) in breast cancer predisposition genes.
Methods
We identified patients from two institutions with germline PVs in high- and moderate-penetrance breast cancer predisposition genes and an HRL in an intact breast, including atypical ductal hyperplasia (ADH), flat epithelial atypia (FEA), and lobular neoplasia (LN). We calculated upgrade rates at surgical excision and used Kaplan–Meier methods to characterize 3-year breast cancer risk in patients without upgrade.
Results
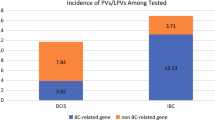
Of 117 lesions in 105 patients, 65 (55.6%) were ADH, 48 (41.0%) were LN, and 4 (3.4%) were FEA. Most PVs (83.8%) were in the BRCA1/2, CHEK2 and ATM genes. ADH and FEA were excised in most cases (87.1%), with upgrade rates of 11.8% (95% confidence interval [CI] 5.5–23.4%) and 0%, respectively. LN was selectively excised (53.8%); upgrade rate in the excision group was 4.8% (95% CI 0.8–22.7%), and with 20 months of median follow-up, no same-site cancers developed in the observation group. Among those not upgraded, the 3-year risk of breast cancer development was 13.1% (95% CI 6.3–26.3%), mostly estrogen receptor-positive (ER +) disease (89.5%).
Conclusions
Upgrade rates for HRLs in patients with PVs in breast cancer predisposition genes appear similar to non-carriers. HRLs may be associated with increased short-term ER+ breast cancer risk in PV carriers, warranting strong consideration of surgical or chemoprevention therapies in this population.
Similar content being viewed by others
References
Schiaffino S, Calabrese M, Melani EF, et al. Upgrade rate of percutaneously diagnosed pure atypical ductal hyperplasia: systematic review and meta-analysis of 6458 lesions. Radiology. 2020;294(1):76–86. https://doi.org/10.1148/radiol.2019190748.
Menes TS, Rosenberg R, Balch S, Jaffer S, Kerlikowske K, Miglioretti DL. Upgrade of high-risk breast lesions detected on mammography in the Breast Cancer Surveillance Consortium. Am J Surg. 2014;207(1):24–31. https://doi.org/10.1016/j.amjsurg.2013.05.014.
Pena A, Shah SS, Fazzio RT, et al. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat. 2017;164(2):295–304. https://doi.org/10.1007/s10549-017-4253-1.
Lustig DB, Guo M, Liu C, et al. Development and prospective validation of a risk calculator that predicts a low risk cohort for atypical ductal hyperplasia upstaging to malignancy: evidence for a watch and wait strategy of a high-risk lesion. Ann Surg Oncol. 2020;27(12):4622–7. https://doi.org/10.1245/s10434-020-08881-0.
Lamb LR, Bahl M, Hughes KS, Lehman CD. Pathologic upgrade rates of high-risk breast lesions on digital two-dimensional vs tomosynthesis mammography. J Am Coll Surg. 2018;226(5):858–67. https://doi.org/10.1016/j.jamcollsurg.2017.12.049.
Rudin AV, Hoskin TL, Fahy A, et al. Flat epithelial atypia on core biopsy and upgrade to cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2017;24(12):3549–58. https://doi.org/10.1245/s10434-017-6059-0.
Liu C, Dingee CK, Warburton R, et al. Pure flat epithelial atypia identified on core needle biopsy does not require excision. Eur J Surg Oncol. 2020;46(2):235–9. https://doi.org/10.1016/j.ejso.2019.10.029.
Hugar SB, Bhargava R, Dabbs DJ, Davis KM, Zuley M, Clark BZ. Isolated flat epithelial atypia on core biopsy specimens is associated with a low risk of upgrade at excision. Am J Clin Pathol. 2019;151(5):511–5. https://doi.org/10.1093/ajcp/aqy175.
Grabenstetter A, Brennan S, Salagean ED, Morrow M, Brogi E. Flat epithelial atypia in breast core needle biopsies with radiologic-pathologic concordance: Is excision necessary? Am J Surg Pathol. 2020;44(2):182–90. https://doi.org/10.1097/PAS.0000000000001385.
Dana-Farber Cancer Institute. The Incidence of Adjacent Synchronous Ipsilateral Infiltrating Carcinoma and/or DCIS in Patients Diagnosed With Intraductal Papilloma Without Atypia or Flat Epithelial Atypia by Core Needle Biopsy. ClinicalTrials.gov. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT02489617?term=NCT02489617&rank=1. Accessed 26 Jun 2023.
Chaudhary S, Lawrence L, McGinty G, Kostroff K, Bhuiya T. Classic lobular neoplasia on core biopsy: a clinical and radio-pathologic correlation study with follow-up excision biopsy. Mod Pathol. 2013;26(6):762–71. https://doi.org/10.1038/modpathol.2012.221.
Rendi MH, Dintzis SM, Lehman CD, Calhoun KE, Allison KH. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol. 2012;19(3):914–21. https://doi.org/10.1245/s10434-011-2034-3.
Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21(10):1208–16. https://doi.org/10.1038/modpathol.2008.134.
Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in-situ in patients with lobular neoplasia on core biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann Surg Oncol. 2016;23(3):722–8. https://doi.org/10.1245/s10434-015-4922-4.
Laws A, Katlin F, Nakhlis F, Chikarmane SA, Schnitt SJ, King TA. Atypical lobular hyperplasia and classic lobular carcinoma in situ can be safely managed without surgical excision. Ann Surg Oncol. 2022;29(3):1660–7. https://doi.org/10.1245/s10434-021-10827-z.
Matar R, Sevilimedu V, Park A, King TA, Pilewskie M. Comparison of outcomes for classic-type lobular carcinoma in situ managed with surgical excision after core biopsy versus observation. Ann Surg Oncol. 2022;29(3):1670–9. https://doi.org/10.1245/s10434-021-10828-y.
Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast–risk assessment and management options. N Engl J Med. 2015;372(1):78–89. https://doi.org/10.1056/NEJMsr1407164.
Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361(9352):125–9. https://doi.org/10.1016/S0140-6736(03)12230-1.
King TA, Pilewskie M, Muhsen S, et al. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol. 2015;33(33):3945–52. https://doi.org/10.1200/JCO.2015.61.4743.
Wong SM, King T, Boileau JF, Barry WT, Golshan M. Population-based analysis of breast cancer incidence and survival outcomes in women diagnosed with lobular carcinoma in situ. Ann Surg Oncol. 2017;24(9):2509–17. https://doi.org/10.1245/s10434-017-5867-6.
NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic. Version 2.2024. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Risk Reduction. Version 1.2024. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf
Braun D, Yang J, Griffin M, Parmigiani G, Hughes KS. A clinical decision support tool to predict cancer risk for commonly tested cancer-related germline mutations. J Genet Couns. 2018;27(5):1187–99. https://doi.org/10.1007/s10897-018-0238-4.
ASK2ME™ (All Syndromes Known to Man Evaluator™). Available at: https://ask2me.org/index.php. Accessed 27 Jun 2023
Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–16. https://doi.org/10.1001/jama.2017.7112.
Nguyen-Dumont T, Dowty JG, Steen JA, et al. Population-based estimates of the age-specific cumulative risk of breast cancer for pathogenic variants in CHEK2: findings from the Australian breast cancer family registry. Cancers (Basel). 2021;13(6):1378. https://doi.org/10.3390/cancers13061378.
Renault AL, Dowty JG, Steen JA, et al. Population-based estimates of age-specific cumulative risk of breast cancer for pathogenic variants in ATM. Breast Cancer Res. 2022;24(1):24. https://doi.org/10.1186/s13058-022-01518-y.
Kauff ND, Brogi E, Scheuer L, et al. Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer. 2003;97(7):1601–8. https://doi.org/10.1002/cncr.11225.
Hoogerbrugge N, Bult P, de Widt-Levert LM, et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol. 2003;21(1):41–5. https://doi.org/10.1200/JCO.2003.02.137.
Cha E, Ambinder EB, Oluyemi ET, et al. High-risk lesions in the breast diagnosed by MRI-guided core biopsy: upgrade rates and features associated with malignancy. Breast Cancer Res Treat. 2022;196(3):517–25. https://doi.org/10.1007/s10549-022-06761-7.
Speer ME, Huang ML, Dogan BE, et al. High risk breast lesions identified on MRI-guided vacuum-assisted needle biopsy: outcome of surgical excision and imaging follow-up. Br J Radiol. 2018;91(1090):20180300. https://doi.org/10.1259/bjr.20180300.
Khoury T, Li Z, Sanati S, et al. The risk of upgrade for atypical ductal hyperplasia detected on magnetic resonance imaging-guided biopsy: a study of 100 cases from four academic institutions. Histopathology. 2016;68(5):713–21. https://doi.org/10.1111/his.12811.
Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23–32. https://doi.org/10.1001/jamaoncol.2014.168.
Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju091. https://doi.org/10.1093/jnci/dju091.
Cybulski C, Wokolorczyk D, Jakubowska A, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29(28):3747–52. https://doi.org/10.1200/JCO.2010.34.0778.
Gallagher S, Hughes E, Kurian AW, et al. Comprehensive breast cancer risk assessment for CHEK2 and ATM pathogenic variant carriers incorporating a polygenic risk score and the Tyrer-Cuzick Model. JCO Precis Oncol. 2021. https://doi.org/10.1200/PO.20.00484.
Metcalfe K, Eisen A, Senter L, et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer. 2019;121(1):15–21. https://doi.org/10.1038/s41416-019-0446-1.
Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62. https://doi.org/10.1093/jnci/dji372.
Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–91. https://doi.org/10.1056/NEJMoa1103507.
Cuzick J, Sestak I, Forbes JF, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. 2020;395(10218):117–22. https://doi.org/10.1016/S0140-6736(19)32955-1.
King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286(18):2251–6.
Kotsopoulos J, Gronwald J, Huzarski T, et al. Tamoxifen and the risk of breast cancer in women with a BRCA1 or BRCA2 mutation. Breast Cancer Res Treat. 2023;201(2):257–64. https://doi.org/10.1007/s10549-023-06991-3.
Breast Cancer Association Consortium, Mavaddat N, Dorling L, et al. Pathology of tumors associated with pathogenic germline variants in 9 breast cancer susceptibility genes. JAMA Oncol. 2022;8(3):e216744. https://doi.org/10.1001/jamaoncol.2021.6744.
Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440–51. https://doi.org/10.1056/NEJMoa2005936.
Tomiczek-Szwiec J, Szwiec M, Falco M, et al. The impact of oophorectomy on survival from breast cancer in patients with CHEK2 mutations. Br J Cancer. 2022;127(1):84–91. https://doi.org/10.1038/s41416-022-01770-1.
Acknowledgments
The authors would like to acknowledge Kevin S. Hughes, MD, and Kanhua Yin, MD, MPH, for their assistance with data collection.
Funding
Rebecca Winn Matchett, Christopher Matchett, and the Winn Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
TAK reports speakers honoraria and advisory board participation for Exact Sciences. SM is Chief Scientific Officer and a founder of INHERET, LLC. The remaining authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laws, A., Leonard, S., Hershey, E. et al. Upgrade Rates and Breast Cancer Development Among Germline Pathogenic Variant Carriers with High-Risk Breast Lesions. Ann Surg Oncol 31, 3120–3127 (2024). https://doi.org/10.1245/s10434-024-14947-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-14947-0