Abstract
Background
The impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) on quality of life (QoL) for patients taking opioids and psychotropic medications preoperatively is unclear.
Methods
This study retrospectively reviewed a CRS-HIPEC single-center prospectively maintained database for 2012–2016. Demographics and clinical data on opioids/psychotropic medication use were collected via chart review. The study collected QoL outcomes at baseline, then 3, 6, and 12 months postoperatively via the Center for Epidemiologic Studies Depression Scale (CES-D), Brief Pain Inventory, Functional Assessment of Cancer Therapy, and 36-Item Short-Form Health Survey. Differences in QoL between the groups were calculated using repeated measures analysis of variance regression. Descriptive statistics and Kaplan-Meier analyses were performed.
Results
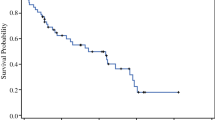
Of 388 patients, 44.8% were taking opioids/psychotropic medications preoperatively. At baseline, those taking opioids/psychotropic medications preoperatively versus those not taking these medications had significantly worse QoL. By 1 year postoperatively, the QoL measures did not differ significantly except for emotional functioning (e.g., no medications vs. opioids/psychotropic medications: CES-D, 5.6 vs. 10.1). Median survival did not differ significantly (opioids/psychotropic medications vs. no medications: 52.3 vs. 60.6 months; p = 0.66). At 1 year after surgery, a greater percentage of patients were taking opioids, psychotropic medications, or both than at baseline (63.2% vs. 44.8%; p < 0.001).
Conclusion
Despite worse baseline QoL, patients who took opioids/psychotropic medications had QoL scores 1 year postoperatively similar to the scores of those who did not except in the emotional domains. These data point to the potential utility of a timed psychosocial intervention to enhance emotional adaptation and further support the role of CRS-HIPEC in improving QoL.

Similar content being viewed by others
References
Levine EA, Stewart JH, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1000 patients. J Am Coll Surg. 2014;218:573–85.
Jafari MD, Halabi WJ, Stamos MJ, Nguyen VQ, Carmichael JC, Mills SD, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the American College of Surgeons national surgical quality improvement program. JAMA Surg. 2014;149:170–5.
Foster JM, Sleightholm R, Patel A, Shostrom V, Hall B, Neilsen B, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw Open. 2019;2:e186847.
Dodson RM, McQuellon RP, Mogal HD, Duckworth KE, Russell GB, Votanopoulos KI, et al. Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(Suppl 5):772–83.
Syrowatka A, Chang SL, Tamblyn R, Mayo NE, Meguerditchian AN. Psychotropic and opioid medication use in older patients with breast cancer across the care trajectory: a population-based cohort study. J Natl Compr Canc Netw. 2016;14:1412–9.
Aalberg JJ, Kimball MD, McIntire TR, McCullen GM. Long-term outcomes of persistent post-operative opioid use: a retrospective cohort study. Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005372.
Sun M, Chang CL, Lu CY, Zhang J, Wu SY. Effect of opioids on cancer survival in patients with chronic pain: a propensity score-matched population-based cohort study. Br J Anaesth. 2022;128:708–17.
Ratcliff CG, Massarweh NN, Sansgiry S, Dindo L, Cully JA. Impact of psychiatric diagnoses and treatment on postoperative outcomes among patients undergoing surgery for colorectal cancer. Psychiatr Serv. 2021;72:391–8.
Rieser C, Phelos H, Zureikat A, Pingpank J, Ongchin M, Lee A, et al. Socioeconomic barriers to CRS HIPEC for appendiceal cancer within a regional academic hospital system. Ann Surg Oncol. 2022;29(11):6593–602.
Sanjida S, Janda M, Kissane D, Shaw J, Pearson SA, DiSipio T, et al. A systematic review and meta-analysis of prescribing practices of antidepressants in cancer patients. Psychooncology. 2016;25:1002–16.
RAND Corporation. 36-Item Short Form Survey (SF-36). Retrieved 8 December 2022 at https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html.
Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ. 2009;338:a3006.
Webster K, Cella D, Yost K. The functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79.
Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality-of-life instrument. Qual Life Res. 1999;8:181–95.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401.
Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–14.
Sanjida S, Janda M, McPhail SM, Kissane D, Couper J, Scott J, et al. How many patients enter endometrial cancer surgery with psychotropic medication prescriptions, and how many receive a new prescription perioperatively? Gynecol Oncol. 2019;152:339–45.
Coyne JC, Palmer SC, Shapiro PJ, Thompson R, DeMichele A. Distress, psychiatric morbidity, and prescriptions for psychotropic medication in a breast cancer waiting room sample. Gen Hosp Psychiatry. 2004;26:121–8.
Chen Y, Spillane S, Shiels MS, Young L, Quach D, Berrington de González A, et al. Trends in opioid use among cancer patients in the United States: 2013–2018. JNCI Cancer Spectr. 2022;6(1):pkab095. https://doi.org/10.1093/jncics/pkab095.
Ng CG, Boks MP, Smeets HM, Zainal NZ, de Wit NJ. Prescription patterns for psychotropic drugs in cancer patients; a large population study in the Netherlands. Psychooncology. 2013;22:762–7.
Wang CH, Huang LC, Yang CC, Chen CL, Chou YJ, Chen YY, et al. Short- and long-term use of medication for psychological distress after the diagnosis of cancer. Support Care Cancer. 2017;25:757–68.
McQuellon RP, Russell GB, Shen P, Stewart JH, Saunders W, Levine EA. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol. 2008;15:125–33.
Leimkühler M, Hentzen J, Hemmer PHJ, Been LB, van Ginkel RJ, Kruijff S, et al. Systematic review of factors affecting quality of life after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2020;27:3973–83.
Zheng J, He J, Wang W, Zhou H, Cai S, Zhu L, et al. The impact of pain and opioids use on survival in cancer patients: results from a population-based cohort study and a meta-analysis. Med Baltim. 2020;99:e19306.
Zylberberg HM, Woodrell C, Rustgi SD, Aronson A, Kessel E, Amin S, et al. Opioid prescription is associated with increased survival in older adult patients with pancreatic cancer in the United States: a propensity score analysis. JCO Oncol Pract. 2022;18:e659–68.
Chino F, Kamal A, Chino J. Incidence of opioid-associated deaths in cancer survivors in the United States, 2006–2016: a population study of the opioid epidemic. JAMA Oncol. 2020;6:1100–2.
Jairam V, Yang DX, Verma V, Yu JB, Park HS. National patterns in prescription opioid use and misuse among cancer survivors in the United States. JAMA Netw Open. 2020;3:e2013605.
Cata JP, Keerty V, Keerty D, Feng L, Norman PH, Gottumukkala V, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–8.
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4.
Scavonetto F, Yeoh TY, Umbreit EC, Weingarten TN, Gettman MT, Frank I, et al. Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: a large, retrospective matched cohort study. Br J Anaesth. 2014;113(Suppl 1):i95-102.
Cummings KC III, Xu F, Cummings LC, Cooper GS. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population-based study. Anesthesiology. 2012;116:797–806.
Owusu-Agyemang P, Hayes-Jordan A, Van Meter A, Williams UU, Zavala AM, Kapoor R, et al. Assessing the survival impact of perioperative opioid consumption in children and adolescents undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Paediatr Anaesth. 2017;27:648–56.
Call TR, Pace NL, Thorup DB, Maxfield D, Chortkoff B, Christensen J, et al. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology. 2015;122:317–24.
Cata JP, Nguyen LT, Ifeanyi-Pillette IC, Van Meter A, Dangler LA, Feng L, et al. An assessment of the survival impact of multimodal anesthesia/analgesia technique in adults undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a propensity score-matched analysis. Int J Hyperthermia. 2019;36:369–75.
Leo Swenne C, Jangland E, Arakelian E. Patients’ experiences of their everyday life 14 months after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a qualitative follow-up study. Scand J Caring Sci. 2017;31:904–13.
Teo I, Krishnan A, Lee GL. Psychosocial interventions for advanced cancer patients: a systematic review. Psychooncology. 2019;28:1394–407.
Hoon LS, Chi Sally CW, Hong-Gu H. Effect of psychosocial interventions on outcomes of patients with colorectal cancer: a review of the literature. Eur J Oncol Nurs. 2013;17:883–91.
Fawzy FI. Psychosocial interventions for patients with cancer: what works and what doesn’t. Eur J Cancer. 1999;35:1559–64.
Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: achievements and challenges. CA Cancer J Clin. 2008;58:214–30.
Vos PJ, Visser AP, Garssen B, Duivenvoorden HJ, de Haes HC. Effects of delayed psychosocial interventions versus early psychosocial interventions for women with early-stage breast cancer. Patient Educ Couns. 2006;60:212–9.
Martin RC II, Marshall BM, Philips P, Egger M, McMasters KM, Scoggins CR. Enhanced recovery after surgery is safe for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am J Surg. 2020;220:1428–32.
Webb C, Day R, Velazco CS, Pockaj BA, Gray RJ, Stucky CC, et al. Implementation of an Enhanced Recovery After Surgery (ERAS) program is associated with improved outcomes in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2020;27:303–12.
Acknowledgment
The authors acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center Biostatistics Shared Resource, supported by the National Cancer Institute’s Cancer Center Support Grant award no. P30CA012197. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors have no disclosures to report. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Presence of documented psychiatric diagnoses among those taking opioids and/or psychotropic (psych) medications (meds) before CRS-HIPEC
Diagnosis | No psych meds (n = 214) n (%) | Opioids (n = 102) n (%) | Anxiolytics (n = 73) n (%) | Antidepressants (n = 73) n (%) | Antipsychotics (n = 4) n (%) | Other psych med (n = 5) n (%) | Any med (n = 174) n (%) | Opioids only (n = 51) n (%) | Psych meds only (n = 72) n (%) | Opioids + psych meds (n = 51) n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
No psych history documented | 209 (97.7) | 72 (70.6) | 44 (60.3) | 27 (37.0) | 2 (50) | 2 (40) | 107 (61.5) | 46 (90.2) | 35 (48.6) | 26 (51) |
Depression | 2 (0.9) | 21 (20.6) | 17 (23.3) | 38 (52.1) | 1 (25) | 2 (40) | 45 (25.9) | 3 (5.9) | 24 (33.3) | 18 (35.3) |
Anxiety | 4 (1.9) | 13 (12.7) | 18 (24.7) | 16 (21.9) | 0 (0) | 1 (20) | 30 (17.2) | 2 (3.9) | 17 (23.6) | 11 (21.6) |
PTSD | 0 (0) | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) | 1 (1.4) | 0 (0) |
Bipolar | 0 (0) | 0 (0)) | 0 (0) | 1 (1.4) | 1 (25) | 1 (20) | 3 (1.7) | 0 (0) | 3 (4.2) | 0 (0) |
Other | 0 (0) | 1 (1) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 1 (2) |
Appendix 2: QoL outcomes for those taking versus those not taking opioids only preoperatively
Instrument | Time | Opioids only (SE) | Not opioids only (SE) | Pairwise comparison (SE) | p value |
|---|---|---|---|---|---|
CES-D | Preop | 14.8 (2.4) | 11.3 (0.8) | 3.5 (2.6) | 0.17 |
3 months | 11.1 (2.9) | 11.4 (0.9) | − 0.3 (3.0) | 0.92 | |
6 months | 12.2 (2.9) | 9.6 (1.0) | 2.6 (3.0) | 0.4 | |
12 months | 12.2 (3.0) | 7.0 (1.0) | 5.1 (3.2) | 0.11 | |
Overall | 12.6 (1.4) | 9.8 (0.5) | 2.8 (1.5) | 0.06 | |
BPI (worst pain) | Preop | 4.2 (0.5) | 3.0 (0.2) | 1.2 (0.5) | 0.03 |
3 months | 3.0 (0.7) | 3.4 (0.2) | − 0.4 (0.7) | 0.6 | |
6 months | 3.2 (0.6) | 2.6 (0.2) | 0.6 (0.7) | 0.4 | |
12 months | 3.8 (0.7) | 2.5 (0.2) | 1.3 (0.7) | 0.07 | |
Overall | 3.6 (0.3) | 2.9 (0.1) | 0.8 (0.3) | 0.02 | |
BPI (least pain) | Preop | 2.4 (0.3) | 1.7 (0.1) | 0.6 (0.3) | 0.05 |
3 months | 1.3 (0.4) | 1.8 (0.1) | − 0.4 (0.4) | 0.31 | |
6 months | 2.1 (0.4) | 1.5 (0.1) | 0.5 (0.4) | 0.22 | |
12 months | 2.1 (0.4) | 1.4 (0.2) | 0.7 (0.5) | 0.13 | |
Overall | 2.0 (0.2) | 1.6 (0.07) | 0.4 (0.2) | 0.05 | |
Functional WB | Preop | 16.7 (1.5) | 20.1 (0.5) | − 3.6 (1.6) | 0.02 |
3 months | 17.2 (1.7) | 18.0 (0.6) | − 0.8 (1.8) | 0.67 | |
6 months | 19.7 (1.7) | 20.8 (0.6) | − 1.0 (1.8) | 0.58 | |
12 months | 20.3 (1.8) | 22.6 (0.6) | − 2.3 (1.9) | 0.24 | |
Overall | 18.3 (0.8) | 20.4 (0.3) | − 2.0 (0.9) | 0.02 | |
Emotional WB | Preop | 15.1 (1.1) | 16.6 (0.4) | − 1.5 (1.2) | 0.20 |
3 months | 17.3 (1.3) | 19.1 (0.4) | − 1.8 (1.3) | 0.19 | |
6 months | 18.8 (1.3) | 19.6 (0.4) | − 0.9 (1.4) | 0.52 | |
12 months | 16.6 (1.3) | 19.9 (0.5) | − 3.3 (1.4) | 0.02 | |
Overall | 17.0 (0.6) | 18.8 (0.2) | − 1.8 (0.7) | 0.0063 | |
Physical WB | Preop | 20.6 (1.3) | 22.4 (0.4) | − 1.8 (1.4) | 0.20 |
3 months | 20.8 (1.6) | 21.0 (0.5) | − 0.2 (1.6) | 0.90 | |
6 months | 21.8 (1.6) | 23.3 (0.5) | − 0.08 (2.3) | 0.97 | |
12 months | 21.9 (1.6)) | 23.9 (0.6) | − 2.0 (1.7) | 0.25 | |
Overall | 21.3 (0.8) | 22.6 (0.3) | − 1.4 (0.8) | 0.08 | |
Social WB | Preop | 23.7 (1.0) | 24.4 (0.3) | − 0.7 (1.0) | 0.5 |
3 months | 23.8 (1.1) | 24.0 (0.4) | − 0.1 (1.2) | 0.9 | |
6 months | 22.9 (1.1) | 23.8 (0.4) | − 0.8 (1.2) | 0.49 | |
12 months | 23.1 (1.2) | 24.4 (0.4)_ | − 1.4 (1.3) | 0.28 | |
Overall | 23.4 (0.6) | 24.1 (0.2) | − 0.7 (0.6) | 0.21 | |
FACT-G | Preop | 75.8 (3.9) | 83.4 | − 7.6 (4.1) | 0.5240 |
3 months | 79.2 (4.5) | 82.0 (1.5) | − 2.8 (4.8) | 0.5564 | |
6 months | 83.3 (4.5) | 87.6 (1.6) | − 4.3 (4.8) | 0.3694 | |
12 months | 81.9 (4.7) | 90.7 (1.7) | − 8.8 (5.0) | 0.0795 | |
Overall | 80.0 (2.2) | 85.9 (0.8) | − 5.9 (2.3) | 0.0104 | |
Cancer subscale | Preop | 18.3 (1.3) | 21.4 (0.4) | − 3.1 (1.4) | 0.0262 |
3 months | 20.2 (1.5) | 19.1 (0.5) | 1.0 (1.6) | 0.5274 | |
6 months | 20.3 (1.5) | 20.9 (0.5) | − 0.6 (1.6) | 0.73 | |
12 months | 20.0 (1.7) | 21.1 (0.6) | − 1.1 (1.8) | 0.5461 | |
Overall | 10.5 (0.8) | 20.7 (0.3) | − 1.1 | 0.1652 | |
FACT-G + subscale | Preop | 94.1 (4.9) | 104.7 (1.6) | − 10.6 (5.1) | 0.04 |
3 months | 99.3 (5.6) | 101.1 (1.9) | − 1.7 (5.9) | 0.8 | |
6 months | 103.6 (5.6) | 108.5 (2.0) | − 4.9 (6.0) | 0.4 | |
12 months | 100.3 (6.2) | 111.9 (2.1) | − 11.6 (6.5) | 0.08 | |
Overall | 99.3 (2.8) | 106.6 (0.9) | − 7.3 (2.9) | 0.01 | |
TOI | Preop | 54.0 (3.1) | 60.3 (1.0) | − 6.4 (3.2) | 0.05 |
3 months | 58.3 (3.5) | 59.1 (1.2) | − 0.9 (3.7) | 0.8 | |
6 months | 60.9 (3.5) | 63.9 (1.2) | − 3.0 (3.8) | 0.4 | |
12 months | 57.8 (3.9) | 65.1 (1.3) | − 7.3 (4.1) | 0.08 | |
Overall | 57.7 (1.7) | 62.1 | − 4.4 (1.8) | 0.02 | |
SF-36 mental | Preop | 34.8 (2.4) | 37.7 (0.8) | − 2.8 (2.6) | 0.27 |
3 months | 38.7 (3.1) | 36.4 (1.0) | 2.3 (3.3) | 0.48 | |
6 months | 38.5 (2.8) | 38.6 (1.1) | − 0.06 (3.0) | 0.98 | |
12 months | 36.6 (2.8) | 39.3 (1.2) | − 2.7 (3.0) | 0.37 | |
Overall | 36.9 (1.4) | 38.0 (0.5) | − 1.1 (1.5) | 0.45 | |
SF-36 physical | Preop | 39.1 (2.5) | 44.9 (0.8) | − 5.8 (2.6) | 0.028 |
3 months | 36.9 (3.2) | 39.2 (1.1) | − 2.3 (3.4) | 0.49 | |
6 months | 41.0 (2.8) | 43.6 (1.1) | − 2.6 (3.1) | 0.4 | |
12 months | 39.5 (2.8) | 43.9 (1.3) | − 4.3 (3.1) | 0.17 | |
Overall | 38.9 (1.4) | 42.9 (0.5) | –4.0 (1.5) | 0.008 |
Appendix 3: QoL outcomes for those taking versus those not taking psychotropic (psych) medications (meds) only preoperatively
Instrument | Time | Not psych meds only (SE) | Psych meds only (SE) | Pairwise comparison (SE) | p value |
|---|---|---|---|---|---|
CES-D | Preop | 10.7 (0.9) | 14.8 (1.6) | − 4.2 (1.8) | 0.02 |
3 months | 11.1 (1.0) | 12.5 (2.1) | − 1.4 (2.3) | 0.6 | |
6 months | 9.8 (1.0) | 10.2 (2.3) | − 0.3 (2.5) | 0.90 | |
12 months | 7.2 (1.1) | 9.1 (2.1) | − 2.0 (2.4) | 0.42 | |
Overall | 9.7 (0.5) | 12.0 (1.0) | − 2.3 (1.1) | 0.04 | |
BPI (worst pain) | Preop | 3.1 (0.2) | 3.1 (0.4) | 0.04 (0.4) | 0.93 |
3 months | 3.4 (0.2) | 2.8 (0.4) | 0.6 (0.5) | 0.26 | |
6 months | 2.8 (0.2) | 2.1 (0.5) | 0.70 (0.6) | 0.2 | |
12 months | 2.8 (0.3) | 2.2 (0.5) | 0.6 (0.6) | 0.28 | |
Overall | 3.0 (0.1) | 2.6 (0.2) | 0.4 (0.3) | 0.11 | |
BPI (least pain) | Preop | 1.9 (0.1) | 1.6 (0.2) | 0.2 (0.3) | 0.34 |
3 months | 1.7 (0.1) | 1.7 (0.3) | − 0.0004 (0.3) | 0.99 | |
6 months | 1.6 (0.2) | 1.8 (0.3) | − 0.2 (0.4) | 0.51 | |
12 months | 1.5 (0.2) | 1.5 (0.3) | 0.02 (0.4) | 0.96 | |
Overall | 1.7 (0.07) | 1.6 (0.1) | 0.05 (0.2) | 0.75 | |
Functional WB | Preop | 19.9 (0.5) | 19.5 (1.0) | 0.4 (1.1) | 0.72 |
3 months | 18.2 (0.6) | 16.9 (1.3) | 1.3 (1.5) | 0.37 | |
6 months | 20.8 (0.6) | 19.9 (1.4) | 1.0 (1.5) | 0.53 | |
12 months | 22.5 (0.7) | 21.8 (1.3) | 0.7 (1.5) | 0.61 | |
Overall | 20.3 (0.3) | 19.6 (0.6) | 0.8 (0.7) | 0.25 | |
Emotional WB | Preop | 16.8 (0.4) | 15.1 (0.7) | 1.7 (0.8) | 0.04 |
3 months | 18.9 (0.4) | 18.9 (1.0) | 0.02 (1.1) | 0.98 | |
6 months | 19.7 (0.5) | 18.8 (1.0) | 0.8 (1.1) | 0.46 | |
12 months | 19.7 (0.5) | 19.0 (0.9) | 0.7 (1.1) | 0.52 | |
Overall | 18.8 (0.2) | 17.8 (0.4) | 0.9 (0.5) | 0.06 | |
Physical WB | Preop | 22.3 (0.5) | 22.3 (0.9) | − 0.03 (1.0) | 0.97 |
3 Months | 21.0 (90.5) | 20.9 (1.2) | 0.10 (1.3) | 0.94 | |
6 Months | 23.1 (0.6) | 23.2 (1.3) | − 0.15 (1.4) | 0.91 | |
12 months | 23.7 (0.6) | 23.6 (1.2) | 0.17 (1.3) | 0.90 | |
Overall | 22.5 (0.3) | 22.5 (0.5) | 0.02 (0.6) | 0.98 | |
Social WB | Preop | 24.2 (0.4) | 24.5 (0.6) | − 0.3 (0.7) | 0.7 |
3 months | 23.7 (0.4) | 24.9 (0.9) | − 1.2 (0.9) | 0.2 | |
6 months | 23.5 (0.4) | 24.5 (0.9) | − 1.0 (1.0) | 0.3 | |
12 months | 24.1 (0.4) | 25.0 (0.8) | − 1.0 (1.0) | 0.3 | |
Overall | 23.9 (0.2) | 24.7 (0.4) | − 0.8 (0.4) | 0.08 | |
FACT-G | Preop | 83.1 (1.4) | 81.4 (2.5) | 1.7 (2.9) | 0.5645 |
3 months | 81.7 (1.6) | 81.6 (3.4) | 0.2 (3.8) | 0.05 | |
6 months | 87.0 (1.6) | 87.4 (3.7) | − 0.4 (4.1) | 0.9 | |
12 months | 89.8 (1.8) | 89.4 (3.4) | 0.5 (3.8) | 0.9 | |
Overall | 85.4 (0.8) | 84.7 (1.6) | 0.7 (1.8) | 0.6998 | |
Cancer subscale | Preop | 21.1 (0.5) | 21.1 (0.9) | 0.08 (1.0) | 0.9382 |
3 months | 19.4 (0.5) | 18.4 (1.7) | 1.0 (1.3) | 0.5 | |
6 months | 21.0 (0.6) | 19.8 (1.2) | 1.3 (1.4) | 0.3 | |
12 months | 20.9 (0.6) | 21.1 (1.1) | − 0.1 (1.3) | 0.9 | |
Overall | 20.6 (0.3) | 20.2 (0.5) | 0.5 (0.6) | 0.4 | |
FACT-G + subscale | Preop | 104.0 (1.8) | 102.4 (3.2) | 1.6 (3.6) | 0.7 |
3 months | 101.1 (2.0) | 100.0 (4.3) | 1.1 (4.7) | 0.8 | |
6 months | 108.05 (2.0) | 107.7 (4.6) | 0.4 (5.1) | 0.9 | |
12 months | 110.8 (2.3) | 110.4 (4.2) | 0.4 (4.8) | 0.9 | |
Overall | 106.0 (1.0) | 105.0 (1.9) | 1.0 (2.2) | 0.7 | |
TOI | Preop | 60.1 (1.1) | 58.4 (2.0) | 1.6 (2.3) | 0.5 |
3 months | 59.2 (1.2) | 58.2 (2.7) | 1.0 (3.0) | 0.7 | |
6 months | 63.8 (1.3) | 62.5 (2.9) | 1.4 (3.2) | 0.7 | |
12 months | 64.5 (1.4) | 63.6 (2.6) | 0.9 (3.0) | 0.8 | |
Overall | 61.9 (0.6) | 60.6 (1.2) | 1.3 (1.4) | 0.4 | |
SF-36 mental | Preop | 38.2 (0.9) | 34.9 (1.5) | 3.3 (1.7) | 0.06 |
3 months | 37.3 (1.1) | 33.0 (2.3) | 4.3 (2.6) | 0.09 | |
6 months | 38.5 (1.1) | 38.6 (2.4) | − 0.07 (2.7) | 0.98 | |
12 months | 39.0 (1.3) | 38.5 (2.2) | 0.05 (2.6) | 0.85 | |
Overall | 38.4 (0.5) | 36.0 (1.0) | 2.36 (1.1) | 0.04 | |
SF-36 physical | Preop | 44.0 (0.9) | 45.3 (1.1) | − 1.3 (1.8) | 0.48 |
3 months | 39.1 (1.1) | 38.4 (2.4) | 0.7 (2.7) | 0.80 | |
6 months | 43.1 (1.2) | 43.9 (2.5) | − 0.9 (2.8) | 0.75 | |
12 months | 42.8 (1.3) | 44.3 (2.3) | − 1.6 (2.7) | 0.56 | |
Overall | 42.2 (0.6) | 43.1 (1.1) | − 0.9 (1.2) | 0.46 |
Appendix 4: QoL outcomes for those taking versus those not taking both opioids and psychotropic (psych) medications (meds) preoperatively
Instrument | Time | Opioids and psych meds only (SE) | Not opioids and psych meds only (SE) | Pairwise comparison (SE) | p value |
|---|---|---|---|---|---|
CES-D | Preop | 16.7 (2.1) | 10.9 (0.8) | 5.8 (2.3) | 0.01 |
3 months | 15.5 (2.3) | 10.7 (1.0) | 4.8 (2.5) | 0.06 | |
6 months | 15.4 (2.4) | 8.9 (1.0) | 6.5 (2.6) | 0.001 | |
12 months | 9.9 (2.7) | 7.3 (1.0) | 2.6 (2.9) | 0.4 | |
Overall | 14.5 (1.2) | 9.4 (0.5) | 5.1 (1.3) | < 0.001 | |
BPI (worst pain) | Preop | 3.8 (0.5) | 3.0 (0.2) | 0.8 (0.5) | 0.13 |
3 months | 3.7 (0.6) | 3.3 (0.2) | 0.4 (0.6) | 0.51 | |
6 months | 3.4 (0.6) | 2.5 (0.2) | 0.9 (0.6) | 0.15 | |
12 months | 2.9 (0.7) | 2.7 (0.3) | 0.3 (0.7) | 0.70 | |
Overall | 3.5 (0.3) | 2.9 (0.1) | 0.6 (0.3) | 0.04 | |
BPI (least pain) | Preop | 2.4 (0.3) | 1.7 (0.1) | 0.6 (0.3) | 0.05 |
3 months | 2.2 (0.4) | 1.6 (0.1) | 0.5 (0.4) | 0.2 | |
6 months | 2.2 (0.4) | 1.5 (0.2) | 0.7 (0.4) | 0.07 | |
12 months | 1.9 (0.4) | 1.4 (0.2) | 0.4 (0.4) | 0.3 | |
Overall | |||||
Functional WB | Preop | 16.6 (1.3) | 20.3 (0.5) | − 3.7 (1.4) | 0.0076 |
3 months | 16.7 (1.4) | 18.2 (0.6) | − 1.4 (1.5) | 0.35 | |
6 months | 17.9 (1.5) | 21.1 (0.6) | − 3.3 (1.6) | 0.04 | |
12 months | 20.8 (1.7) | 22.6 (0.6) | − 1.8 (1.8) | 0.33 | |
Overall | 17.9 (0.7) | 20.5 (0.3) | − 2.7 (0.8) | 0.0007 | |
Emotional WB | Preop | 14.5 (0.9) | 16.7 (0.4) | − 2.2 (1.0) | 0.03 |
3 months | 18.5 (1.0) | 19.0 (0.4) | − 0.5 (1.1) | 0.65 | |
6 months | 18.4 (1.1) | 19.7 (0.5) | − 1.3 (1.2) | 0.26 | |
12 months | 19.1 (1.3) | 19.6 (0.5) | − 0.5 (1.4) | 0.7 | |
Overall | 17.5 (0.5) | 18.8 (0.2) | − 1.3 (0.6) | 0.03 | |
Physical WB | Preop | 18.8 (1.1) | 22.8 (0.4) | − 4.0 (1.2) | 0.001 |
3 months | 19.3 (1.2) | 21.2 (0.5) | − 2.0 (1.4) | 0.14 | |
6 months | 19.8 (1.3) | 23.7 (0.5) | − 3.9 (1.4) | 0.005 | |
12 months | 21.7 (1.5) | 24.0 (0.6) | − 2.3 (1.6) | 0.15 | |
Overall | 19.8 (0.6) | 22.9 (0.3) | − 3.1 (0.7) | < 0.0001 | |
Social WB | Preop | 24.1 (0.8) | 24.3 (0.3) | − 0.3 (0.9) | 0.8 |
3 months | 23.3 (0.9) | 24.1 (0.4) | − 0.8 (1.0) | 0.5 | |
6 months | 23.3 (1.0) | 23.7 (0.4) | − 0.4 (1.0) | 0.7 | |
12 months | 24.1 (1.1) | 24.3 (0.4) | − 0.2 (1.2) | 0.9 | |
Overall | 23.7 (0.5) | 24.1 (0.2) | − 0.4 (0.5) | 0.4 | |
FACT-G | Preop | 73.9 (3.3) | 84.0 (1.3) | − 10.1 (3.6) | 0.0047 |
3 months | 77.8 (3.7) | 82.4 (1.5) | − 4.6 (4.0) | 0.2435 | |
6 months | 79.4 (3.8) | 88.5 (1.6) | − 9.0 (4.1) | 0.0274 | |
12 months | 87.0 (4.5) | 90.1 (1.7) | − 3.1 (4.8) | 0.5199 | |
Overall | 79.1 (1.9) | 86.3 (0.8) | − 7.2 (2.0) | 0.004 | |
Cancer subscale | Preop | 19.6 (1.1) | 21.4 (0.5) | − 1.8 (1.2) | 0.1 |
3 months | 18.7 (1.3) | 19.3 (0.5) | − 0.7 (1.4) | 0.6 | |
6 months | 19.8 (1.3) | 21.0 (0.5) | − 1.2 (1.4) | 0.4 | |
12 months | 19.9 (1.5) | 21.1 (0.6) | − 1.2 (1.6) | 0.5 | |
Overall | 19.5 (0.6) | 20.7 (0.3) | − 1.3 (0.7) | 0.07 | |
FACT-G + subscale | Preop | 93.5 (4.1) | 105.2 (1.6) | − 11.8 (4.5) | 0.009 |
3 months | 96.4 (4.6) | 101.7 (1.9) | − 5.3 (5.0) | 0.3 | |
6 months | 99.2 (4.7) | 109.6 (2.0) | − 10.3 (5.1) | 0.04 | |
12 months | 107.4 (5.6) | 111.2 (2.1) | 6.0 (− 3.8) | 0.5 | |
Overall | 98.7 (2.3) | 107.0 (1.0) | − 8.3 (2.5) | 0.001 | |
TOI | Preop | 52.8 (2.6) | 60.8 (1.0) | − 7.9 (2.8) | 0.005 |
3 months | 56.4 (2.9) | 59.5 (1.2) | − 3.1 (3.1) | 0.32 | |
6 months | 58.1 (3.0) | 64.6 (1.3) | − 6.5 (3.2) | 0.04 | |
12 months | 62.2 (3.5) | 64.6 (1.3) | − 2.4 (3.8) | 0.5 | |
Overall | 57.0 (1.5) | 62.4 (0.6) | − 5.4 (1.6) | 0.0008 | |
SF-36 mental | Preop | 34.3 (1.9) | 38.0 (0.8) | − 3.7 (2.1) | 0.08 |
3 months | 34.5 (2.3) | 37.0 (1.1) | − 2.6 (2.6) | 0.31 | |
6 months | 35.1 (2.5) | 39.2 (1.1) | − 4.1 (2.7) | 0.14 | |
12 months | 37.8 (2.9) | 39.1 (1.2) | − 1.2 (3.1) | 0.70 | |
Overall | 35.3 (1.2) | 38.3 (0.5) | − 3.1 (1.3) | 0.02 | |
SF-36 physical | Preop | 40.4 (2.0) | 45.1 (0.9) | − 4.7 (2.2) | 0.03 |
3 months | 38.1 (2.4) | 39.2 (1.1) | − 1.1 (2.7) | 0.67 | |
6 months | 42.9 (2.6) | 43.3 (1.2) | − 0.4 (2.9) | 0.9 | |
12 months | 43.0 (3.0) | 43.2 (1.3) | − 0.2 (3.3) | 0.95 | |
Overall | 40.6 (1.2) | 42.8 (0.5) | − 2.1 (1.3) | 0.11 |
Appendix 5
See Fig. 2.
Appendix 6
See Fig. 3.
Appendix 7
See Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solsky, I., Patel, A., Valenzuela, C.D. et al. Quality-of-Life Outcomes for Patients Taking Opioids and Psychotropic Medications Before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 31, 577–593 (2024). https://doi.org/10.1245/s10434-023-14215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14215-7