Abstract
Background
The efficacy, toxicity, and patterns of failure of esophageal squamous cell carcinoma (ESCC) treated with selective lymph node (SLN) conventional fraction radiotherapy (CFRT) and S-1 plus cisplatin (CDDP) were evaluated.
Patients and Methods
67 Patients with clinical stage II–IVa ESCC were enrolled. The total dose of SLN CFRT was 60 Gy in 30 fractions over 6 weeks. The first course of radiation covered the primary and metastatic regional tumors and high-risk lymph nodal regions, given at 2 Gy/fraction for a dose of 40 Gy. In the second course, CFRT was delivered to the boost volume for an additional 20 Gy in 10 days, using 2 Gy/fraction. Two cycles of chemotherapy were given at the beginning of radiotherapy. CDDP at 25 mg/m2/day was given on days 1–3 and days 22–24, and S-1 at 80 mg/m2/day on days 1–14 and days 22–35. Patients achieving objective response after concurrent chemoradiotherapy underwent two additional cycles of chemotherapy.
Results
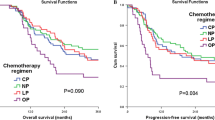
The objective response rate (ORR) was 82.5%. Grade 3 or 4 toxicities included leukopenia (23.8%), neutropenia (14.3%), thrombocytopenia (14.3%), hemoglobin (4.8%), gastrointestinal (12.7%), skin (1.6%), and esophagus fistula (1.6%). One patient died of severe pneumonia, and two died of late toxicity because of esophagus fistula. With median follow-up of 32 months, the overall survival (OS) and progression-free survival (PFS) at 1 year and 2 years were 81.0% and 73.0%, and 63.5% and 49.2%, respectively.
Conclusions
SLN RT concurrent with S-1 plus CDDP may represent a better strategy for treatment of ESCC patients.

Similar content being viewed by others
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66(2):115–32.
Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–74.
Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999; 281(17):1623–7.
Hirata K, Horikoshi N, Aiba K, et al. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res. 1999;5(8):2000–5.
Iwase H, Shimada M, Tsuzuki T, et al. Concurrent chemoradiotherapy with a novel fluoropyrimidine, S-1, and cisplatin for locally advanced esophageal cancer: long-term results of a phase II trial. Oncology. 2013;84(6):342–9.
Cho SH, Shim HJ, Lee SR, et al. Concurrent chemoradiotherapy with S-1 and cisplatin in advanced esophageal cancer. Dis Esophagus. 2008;21(8): 697–703.
Li BS, Zhou T, Wang ZT, et al. Phase I study of concurrent selective lymph node late course accelerated hyper-fractionated radiotherapy and capecitabine, cisplatin for locally advanced esophageal squamous cell carcinoma. Radiother Oncol. 2009; 93(3):458–61.
Wang D, Yang J, Zhu J, et al. Elective lymph node irradiation late course accelerated hyper-fractionated radiotherapy plus concurrent cisplatin-based chemotherapy for esophageal squamous cell carcinoma: a phase II study. Radiat Oncol. 2013; 8(1):108.
Li BS, Gong HY, Huang W, et al. Phase I study of concurrent selective lymph node late course accelerated hyper-fractionated radiotherapy and pemetrexed, cisplatin for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2011; 24(4):251–7.
Li M, Fu C, Zhang W, et al. Phase I study of concurrent selective lymph node late-course accelerated hyperfractionated radiotherapy and S-1 plus cisplatin for locally advanced oesophageal squamous cell carcinoma. Br J Radiol. 2016;89(1060): 20150476.
Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg Oncol. 1994; 220(3):364–73.
Natsugoe S, Matsumoto M, Okumura H, et al. Initial metastatic, including micrometastatic, sites of lymph nodes in esophageal squamous cell carcinoma. J Surg Oncol. 2005; 89(1):6–11.
Li H, Zhang Y, Cai H, et al. Pattern of lymph node metastases in patients with squamous cell carcinoma of the thoracic esophagus who underwent three-field lymphadenectomy. Eur Surg Res. 2007;39(1):1–6.
Isono K, Uchida Y, Watanabe H, et al. Guidelines for clinical and pathologic studies on carcinoma of the esophagus ninth edition. Preface general principles part I. Esophagus. 2004;1(2):61–88.
Sai H, Mitsumori M, Yamauchi C, et al. Concurrent chemoradiotherapy for esophageal cancer: comparison between intermittent standard-dose cisplatin with 5-fluorouracil and daily low-dose cisplatin with continuous infusion of 5-fluorouracil. Int J Clin Oncol. 2004; 9(3):149–53.
Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011; 81(3):684–90.
Tahara M, Fuse N, Mizusawa J, et al. Phase I/II trial of chemoradiotherapy with concurrent S-1 and cisplatin for clinical stage II/III esophageal carcinoma (JCOG 0604). Cancer Sci. 2015;106(10):1414–20.
Acknowledgement
This study was supported by grants from the Key Scientific and Technological Innovation Project of Shandong Province, China (No. 2017CXZC1206), National Natural Science Foundation of China (No. 81874224), National Natural Science Foundation of China (No. 81773232), and the National Key Research and Development Projects of China (No. 2016YFC0904700).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, X., Li, D. et al. Concurrent Selective Lymph Node Radiotherapy and S-1 Plus Cisplatin for Esophageal Squamous Cell Carcinoma: A Phase II Study. Ann Surg Oncol 26, 1886–1892 (2019). https://doi.org/10.1245/s10434-019-07264-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07264-4