Abstract
Background
Preoperative radiotherapy tends to be more frequently used for patients with adenocarcinoma of the esophagogastric junction (AEG); however, the prognostic values of postoperative pathologic characteristics in these patients remain unclear. This study aimed to examine the outcomes in Siewert type II AEG patients receiving preoperative radiotherapy to identify the predictive factors for overall survival (OS).
Methods and Results
A total of 1818 AEG patients undergoing preoperative radiotherapy were reviewed. Univariate analyses showed that age, sex, histology, tumor grade, positive lymph node (PLN), lymph node ratio, and log odds of positive lymph nodes (LODDS) were significantly correlated with OS; however, only age, grade, PLN, and LODDS were identified as independent risk factors in a multivariate regression model. Subsequently, patients were randomly grouped into training and validation cohorts (1:1 ratio), and the beta coefficients of these variables in the training set were used to generate the nomogram. The composite nomogram showed improved prognostic accuracy in the training, validation, and entire cohorts compared with that of TNM stage alone.
Conclusions
In conclusion, our proposed nomogram represents a promising tool for estimating OS in Siewert type II AEG patients after preoperative radiotherapy.
Similar content being viewed by others
The incidence of adenocarcinoma of the esophagogastric junction (AEG) has increased dramatically in both Western and Asian countries over the past several decades.1,2,–3 Complete surgical removal represents the only curative treatment for patients with AEG.4,5 The surgical requirements for an eligible resection include surgery with curative intent and en bloc resection of the tumor with both macroscopically and microscopically negative margins (R0 resection). However, at diagnosis, most Siewert type II AEG tumors have infiltrated deep into the gastric wall and metastasized to the lymph nodes (LNs) and distant organs, i.e. advanced carcinoma.6,7,–8 Under such circumstances, various treatment regimens attempting to improve patient survival, including preoperative radiotherapy, have been developed.
The survival impact of Siewert type II AEG in goblet cell carcinoma (GCC) has been explored in several studies.9,10,11,12,–13 Although few studies have been statistically powered and the survival benefit remains controversial, preoperative radiotherapy combined with chemotherapy rather than preoperative chemotherapy alone is more effective for achieving good pathologic response.14 Indeed, preoperative radiotherapy can achieve clinical downstaging before resection and can reduce the burden of residual microscopic disease at surgery, thus increasing the possibility of R0 resection.15,16 As a result, the combination of preoperative radiotherapy and chemotherapy tends to be more frequently used, rather than chemotherapy alone, for advanced Siewert type II AEG patients in the US.17 However, the prognostic values of various postoperative pathologic characteristics in Siewert type II AEG patients undergoing preoperative radiotherapy remain unclear. Hence, the objective of this retrospective study from the Surveillance, Epidemiology, and End Results (SEER) database was to identify the factors that affect overall survival (OS) in Siewert type II AEG patients receiving preoperative radiotherapy. Moreover, we also aimed to identify the best staging systems for determining the prognosis of these patients.
Materials and Methods
Patients
Data were obtained from the SEER public database. In the 18 SEER registries, the 2016 release of the public use dataset from 1998 and 2013 was queried to identify Siewert type II AEG patients with microscopic diagnostic confirmation. Although the SEER database did not provide detailed information on the Siewert type classification for AEG, the combined selection terminology of ‘Primary Site’ encoded 160 (Cardia) and ‘CS site-specific factor 25’ encoded 982 (esophagus, gastroesophageal junction), allowed us to obtain the Siewert type II AEG.18 Patients were included if they were M0 stage and had received preoperative radiotherapy, surgery, and pathologic examination of the LNs. The National Cancer Institute’s SEER*Stat software (Surveillance Research Program, National Cancer Institute SEER*Stat software [www.seer.cancer.gov/seerstat], Version 8.1.5) was used to access the database.
Demographic and Clinicopathological Variables
Patient demographic and clinicopathological variables were retrieved from the SEER database. The LN metastasis variables we analyzed in the present study included the positive LN (PLN) count, LN ratio (LNR) value, and log odds of positive LNs (LODDS) value. The LNR value was defined as the PLN count divided by the total number of examined LNs (ELNs),19,20 while the LODDS value was defined as loge ([PLN + 0.5]/[negative LN + 0.5]).21,22 The classification intervals of the PLN count were consistent with the N classification in the 8th edition of the American Joint Committee on Cancer (AJCC) staging system,23,24 stratified as ypN0 (no PLNs), ypN1 (one to two PLNs), ypN2 (three to six PLNs), and ypN3 (more than seven PLNs). The final subgroup definitions of the demographic and clinicopathological variables are shown in electronic supplementary Table S1.
Nomograms were established based on multivariate Cox regression coefficients, and calibration plots were generated to compare the expected and observed survival probabilities. Decision curve analyses (DCAs) were performed to assess the clinical utility of prediction models by quantifying the net benefits when different threshold probabilities were considered. In general, the strategy with the highest net benefit at any given risk threshold was considered to have the highest clinical value.
Statistical Analysis
All statistical analyses were conducted using R software (R Foundation for Statistical Computing, Vienna, Austria) and SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA). Missing values were handled by complete case analyses. Group comparisons were performed for continuous and categorical variables using the one-way analysis of variance (ANOVA) and Chi square test, respectively. The secondary endpoint was OS, defined as the period from diagnosis to death due to any reason. The univariate and multivariate analyses were performed using the Cox regression model 30 with ‘Forward LR’ methods to investigate which clinicopathological factors significantly correlated with OS. Among these factors, age, sex, ethnicity, ypT category, tumor grade, and tumor histology type were entered into the regression models as categorical variables; the PLN, LNR, and LODDS were analyzed in the form of continuous variables. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, with an HR < 1.0 indicating a survival benefit. The Cox regression coefficients were employed to generate nomograms, and discrimination of the prognostic models was measured and compared using Harrell’s concordance index (C-index) with the ‘survival’ package. Calibration plots were generated to compare the expected and observed survival probabilities. DCAs were performed to assess the clinical utility of prediction models by quantifying the net benefits when different threshold probabilities were considered. In general, the strategy with the highest net benefit at any given risk threshold was considered to have the highest clinical value. Akaike Information Criterion (AIC) analyses25,26 were used to compare the performances among different LN classifications and staging systems. A smaller AIC value indicated a more desirable model for predicting the outcome. All statistical tests performed were two-sided and p values < 0.05 were considered statistically significant. This study was conducted and reported in line with the Transparent Reporting of a multivariate prediction model for Individual Prediction or Diagnosis (TRIPOD) guidelines.
Results
Patient Selection and Characteristics
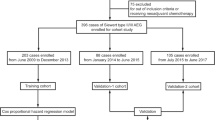
The patient selection schema is shown in Fig. 1. Overall, a total of 1818 patients were included in this study (see electronic supplementary Table S2 for detailed patient characteristics). The median age at diagnosis was 62.0 years (range 24–86). Throughout the follow-up period, there were 997 deaths, 738 of which were attributable to GCC.
Survival Analysis of Postoperative Prognostic Factors
In the univariate analyses of clinicopathological variables as prognostic factors for OS, age, sex, tumor grade, histology type, and all three LN-related factors (PLN, LNR, and LODDS classifications) were significant risk factors for Siewert type II AEG patients after preoperative radiotherapy, whereas the ypT category did not correlate with OS (Table 1). In multivariate analyses of all patients (Table 1), only age, tumor grade, PLN count, and LODDS value were significantly associated with OS.
Characteristics of Patients in the Training and Validation Cohorts
Patients were randomly split into derivation and validation sets based on a 6:4 ratio. Comparison of these two patient groups showed no significant baseline differences (all p > 0.05) (Table 2). The training group was used to evaluate the prognostic effect of clinicopathological variables to determine the beta coefficients (β) (Table 3). Age > 65 years compared with age ≤ 65 years (β = 0.24, p < 0.01), grade 3–4 compared with grade 1–2 (β = 0.27, p < 0.01), PLN number (β = 0.03, p = 0.06), and LODDS value (β = 0.15, p < 0.01) were significantly associated with a decreased OS (Tables 1 and 3), which were used to generate the nomogram.
Construction, Comparison, and Validation of the Nomogram
The nomogram can be used to provide a quantitative method to predict the probability of patient survival. As shown in Fig. 2, the nomogram indicated that the LODDS value was the largest contributor to prognosis, followed, in descending order, by PLN number, age, and Lauren classification. Each value or subtype within these variables was assigned a score on the points scale. By calculating the total score and locating this value on the total points scale, we were able to easily determine the estimated probability of 2-, 3-, and 5-year survival. The forest plots (Fig. 3) revealed that the nomogram could identify patients with different prognoses in either the training cohort, validation cohort, or entire cohort, in each TNM stage subgroup, regardless of the number of LNs resected or the site involved in the surgery. The calibration plots depicted in Fig. 4a–c and Fig. S1a–f showed that the derived nomogram performed well when compared with the performance of an ideal model using the training cohort, validation cohort, or entire cohort. Similarly, using DCA (Fig. 4d and e, and Fig. S2a–f), the nomogram also showed higher net benefit than TNM stage across a range of risk thresholds. We also compared the Harrell’s C-index of the nomogram with that of TNM staging. In the training cohort (Table 4), the C-index for the established nomogram for predicting OS in patients was significantly higher than that of the TNM staging system. Additionally, the AIC value of the nomogram also showed a higher prognostic value than TNM stage (Table 4), indicating that the nomogram, considering age, tumor grade, PLN, and LODDS, was the most effective tool in determining the prognosis of GCC patients who underwent preoperative radiotherapy. Similar results were also observed in the validation cohort as well as the entire cohort (Table 4).
Nomogram construction in the training cohort. Nomogram for predicting the proportion of patients with 2-, 3-, and 5-year overall survival after the diagnosis of adenocarcinoma of the esophagogastric junction, based on four variables (age, grade, PLN and LODDS). The points for each variable are summed to give a total score from which the probability is estimated. PLN positive lymph node, LODDS log odds of positive lymph nodes
Calibration plots and decision curve analyses of the nomogram in the training cohort. a–c Plots depict the calibration of nomograms in terms of agreement between predicted and observed 2-, 3-, and 5-year outcomes in the training cohort. d–f Decision curve analyses of the nomogram and TNM stage for 2-, 3-, and 5-year risk in the training cohort
Discussion
As patients with Siewert type II AEG have poor prognosis, investigation of novel therapeutic strategies, such as neoadjuvant chemotherapy with or without radiation, may be a reasonable approach to improve the management of these patients.27,28,29,30,–31 The prognostic values of post-therapy clinicopathological factors in Siewert type II AEG patients undergoing preoperative radiotherapy have been investigated in several previous studies;14,32,33,34,–35 however, these studies drew conflicting conclusions and all included both non-cardia (i.e. whole gastric) and cardia gastric cancer patients. To date, no studies have specifically focused on Siewert type II AEG patients. For example, in 2014, Orditura et al.34 analyzed 41 patients with AEG who underwent preoperative radiotherapy. With 54 months of follow-up, clinical response and postoperative TNM staging were demonstrated to be the only independent variables related to long-term survival. Ott et al.35 identified ypT category as an independent predictor in all AEG patients undergoing preoperative radiotherapy, as well as in patients classified as R0 responders, whereas ypN category did not maintain statistical significance in multivariate analysis. Furthermore, Gaca et al.33 reported that no variables were significantly associated with OS, and found that pathologic LN status was the only significant predictor of disease-free survival; however, the samples of both studies were small and lacked multivariate analyses.
To our knowledge, the present study is the first review of a large data set from a national cancer registry to explore the survival impact of postoperative clinicopathological factors in Siewert type II AEG patients undergoing preoperative radiotherapy. We found that ypT classification did not reach statistical significance in either univariate or multivariate analyses. Therefore, the AJCC staging system, which is based on depth of invasion and nodal status, might not be applicable for Siewert type II AEG patients after preoperative radiotherapy. In contrast, histology grade and postoperative LN status (PLN number and LODDS) were the only independent prognosis factors correlating with OS. Based on these findings, by integrating the beta coefficients of these independent variables, we established a nomogram for prognostic assessment in Siewert type II AEG patients after preoperative radiotherapy. We observed that the nomogram had a significantly better prognostic value than TNM stage alone.
LODDS is a newly reported index that is considered important and promising for prognosis assessment. The superiority of LODDS as a prognostic classification has been validated in various malignancies, including colorectal cancer, esophageal cancer, breast cancer, etc.36 In Siewert type II AEG, Xu18 performed a population-based study using the SEER database, suggesting that LODDS showed more accurate prognostic performance than PLN and LNR for post-surgery Siewert type II AEG, and could help in detecting survival heterogeneity for patients with no positive LNs involved. In agreement with these previous studies, we found that LODDS was also an independent factor for Siewert type II AEG patients who received preoperative radiotherapy. No doubt our findings further broaden the scope of the clinical use of LODDS.
Our study has some limitations. First, information regarding Siewert types I and III AEG were not available from the SEER database, which limited further analysis of other cancer subtypes. Second, it has been previously reported that clinical response to neoadjuvant therapy is another independent risk factor for survival after curative resection of AEG;35,37 however, the tumor status of patients diagnosed prior to undergoing preoperative radiotherapy was not recorded in the SEER database, thus we were unable to estimate the response rate in our study. Additionally, information regarding other significant risk factors, such as gastrectomy surgical margin status and chemotherapy conduction, was also missing. Despite these limitations, we are confident that our findings will help elucidate the prognostic value of the proposed nomogram in Siewert type II AEG patients who have received preoperative radiotherapy.
Conclusions
The present study demonstrated that the novel nomogram is a better prognostic determinant than other available staging systems in Siewert type II AEG patients after preoperative radiotherapy. This model could enable clinicians to estimate the survival of Siewert type II AEG patients in a more precise fashion. Further prospective studies are required to validate our results.
References
Zhao, J, Zhao, J, Du F, et al. Cardia and non-cardia gastric cancer have similar stage-for-stage prognoses after R0 resection: a large-scale, multicenter study in China. J Gastrointest Surg. 2016;20(4):700–7.
Carr JS, Zafar SF, Saba N, et al. Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer. 2013;44(2):143–51.
Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):235–56.
Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49.
Saedi HS, Mansour-Ghanaei F, Joukar F, et al. Neoadjuvant chemoradiotherapy in non-cardia gastric cancer patients–does it improve survival? Asian Pac J Cancer Prev. 2014;15(20):8667–71.
Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90(2):150–5.
Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States). Cancer Causes Control. 2006;17(7):971–81.
Huang Q, Sun Q, Fan XS, et al. Recent advances in proximal gastric carcinoma. J Dig Dis. 2016;17(7):421–32.
Keller SM, Ryan LM, Coia LR, et al. High dose chemoradiotherapy followed by esophagectomy for adenocarcinoma of the esophagus and gastroesophageal junction: results of a phase II study of the Eastern Cooperative Oncology Group. Cancer. 1998;83(9):1908–16.
Pepek JM, Chino JP, Willett CG, et al. Preoperative chemoradiotherapy for locally advanced gastric cancer. Radiat Oncol. 2013;8:6.
Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009; 27(6):851–6.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84.
Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC): report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42(5):929–34.
An JY, Kim HI, Cheong JH, et al. Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J. 2013;54(4):888–94.
Newton AD, Datta J, Loaiza-Bonilla A, et al. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015;6(5):534–43.
Shridhar R, Dombi GW, Finkelstein SE, et al. Improved survival in patients with lymph node-positive gastric cancer who received preoperative radiation: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2011;117(17):3908–16.
Hingorani M, Crosby T, Maraveyas A, et al. Neoadjuvant chemoradiotherapy for resectable oesophageal and gastro-oesophageal junction cancer: Do we need another randomised trial? Clin Oncol (R Coll Radiol). 2011;23(10):696–705.
Xu J, Cao J, Wang L, et al. Prognostic performance of three lymph node staging schemes for patients with Siewert type II adenocarcinoma of esophagogastric junction. Sci Rep. 2017;7(1):10123.
Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255(3):478–85.
Zhou R, Wu Z, Zhang J, et al. Clinical significance of accurate identification of lymph node status in distant metastatic gastric cancer. Oncotarget. 2016;7(1):1029–41.
Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS ONE. 2012;7(2):e31736.
Wu SG, Sun JY, Yang LC, et al. Prognosis of patients with esophageal squamous cell carcinoma after esophagectomy using the log odds of positive lymph nodes. Oncotarget. 2015;6(34):36911–22.
Nam SH, Bae MR, Roh JL, et al. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol. 2018;87:158–64.
Berger DMS, Wassenberg RM, Jóźwiak K, et al. Inter-observer variation in the histopathology reports of head and neck melanoma; a comparison between the seventh and eighth edition of the AJCC staging system. Eur J Surg Oncol. 2019;45(2):235–41.
Kee KM, Wang JH, Lee CM, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120(12):2650–5.
Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Hepatology. 2001;34(3):529–34.
Deng M, Zeng C, Lu X, et al. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403:175–85.
Wu Y, Pan M, Cui S, et al. Autophagic death induced by thermo–chemotherapy in gastric cancer cells results from the reactive oxygen species pathway. Mol Med Rep. 2016;14(2):1210–8.
Peng H, You K, Zhang R, et al. Predictive value of APAF-1 and COX-2 expression in pathologic complete response to neoadjuvant chemoradiotherapy for patients with locally advanced rectal adenocarcinoma. Oncotarget. 2016;7(23):35233–40.
Huang J, Xu X, Chen H, Yin W, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis. 2013;5(Suppl 3):S267–73.
Li W, Stocchi L, Cherla D, Liu G, et al. Factors associated with hospital readmission following diverting ileostomy creation. Tech Coloproctol. 2017;21(Suppl 3):641–8.
Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23(6):1237–44.
Gaca JG, Petersen RP, Peterson BL, et al. Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Ann Surg Oncol. 2006;13(3):340–6.
Orditura M, Galizia G, Di Martino N, et al. Effect of preoperative chemoradiotherapy on outcome of patients with locally advanced esophagogastric junction adenocarcinoma-a pilot study. Curr Oncol. 2014;21(3):125–33.
Ott K, Blank S, Becker K, et al. Factors predicting prognosis and recurrence in patients with esophago-gastric adenocarcinoma and histopathological response with less than 10% residual tumor. Langenbecks Arch Surg. 2013;398(2):239–49.
Zhou R, Zhang J, Sun H, et al. Comparison of three lymph node classifications for survival prediction in distant metastatic gastric cancer. Int J Surg. 2016;35:165–71.
Lowy AM, Mansfield PF, Leach SD, et al. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229(3):303–8.
Funding
This work was supported by Guangdong Planned Project of Science and Technology (2014A020221001) and Guangzhou Planned Project of Science and Technology (201704020105).
Author information
Authors and Affiliations
Contributions
GZ, RZ, and FJ participated in the study design and coordination and analysis of the results. GZ wrote the manuscript; FL obtained funding and supervised the study; and GL and KL assisted with the English revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Feng Liu, Rui Zhou, Funeng Jiang, Guolong Liu, Kangbao Li, and Guodong Zhu declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. No ethics approval was declared because the SEER is a publicly available database.
Informed Consent
Extraction of data from the publicly available SEER database does not require informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, F., Zhou, R., Jiang, F. et al. Proposal of a Nomogram for Predicting Survival in Patients with Siewert Type II Adenocarcinoma of the Esophagogastric Junction After Preoperative Radiation. Ann Surg Oncol 26, 1292–1300 (2019). https://doi.org/10.1245/s10434-019-07237-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07237-7