Abstract
Background
Tumor-specific fluorescent antibodies, which can be recognized at a cellular or tissue level using optical imaging such as confocal laser endomicroscopy (CLE), could provide a means for rapid and accurate tumor diagnosis and staging. The aim of this study was to evaluate the ability of CLE to detect the presence of tagged cells within lymph nodes in an original simulated metastatic model.
Materials and Methods
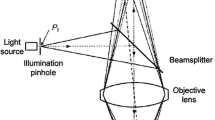
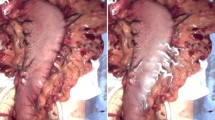
A solution of indocyanine green containing a suspension of porcine hepatocytes, marked with carboxy-fluorescein-succinimidyl-ester (CFSE), was injected endoscopically in the gastric submucosa of 10 pigs. Fluorescence lymphography using a near-infrared laparoscope was used to identify sentinel and secondary drainage nodes. Additionally, a nonfluorescent gastric and a mesenteric node were identified. Every 5–10 min, those nodes were scanned using probe-based or needle-based CLE (pCLE or nCLE). Immunohistochemistry (IHC) using anti-cytokeratin 18 antibodies was subsequently performed to confirm the presence of hepatocytes in the lymph nodes.
Results
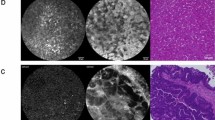
A total of 36 lymph nodes were analyzed with both CLE probes. Hepatocyte penetration in lymph nodes, as assessed by repeated CLE scanning, took 10–40 min after submucosal injection. Concordance between CLE and IHC was 84 and 72 % for pCLE and nCLE, respectively. False negatives were partly due to incomplete CFSE labeling of hepatocytes, which could not be recognized by CLE, but were detected with IHC.
Conclusions
Real-time CLE analysis effectively recognized the presence in perigastric nodes of marked hepatic cells that had been injected endoscopically in the stomach. Validation studies on tumor-bearing animals using tumor-specific antibodies should be performed.




Similar content being viewed by others
References
Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9.
Lee JH, Lee MS, Kim HH, Park DJ, Lee KH, Hwang JY, et al. Feasibility of laparoscopic partial gastrectomy with sentinel node basin dissection in a porcine model. Surg Endosc. 2011;25:1070–5.
Arigami T, Uenosono Y, Yanagita S, Nakajo A, Ishigami S, Okumura H, et al. Clinical significance of lymph node micrometastasis in gastric cancer. Ann Surg Oncol. 2013;20:515–21.
Diana M, Dallemagne B, Chung H, Nagao Y, Halvax P, Agnus V, et al. Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc. 2014;28:3224–33.
Becker V, van den Broek FJ, Buchner AM, Dekker E, Wallace MB, von Delius S, et al. Optimal fluorescein dose for intravenous application in miniprobe-based confocal laser scanning microscopy in pigs. J Biophotonics. 2011;4:108–13.
Metildi CA, Felsen CN, Savariar EN, Nguyen QT, Kaushal S, Hoffman RM, et al. Ratiometric Activatable cell-penetrating peptides label pancreatic cancer, enabling fluorescence-guided surgery, which reduces metastases and recurrence in orthotopic mouse models. Ann Surg Oncol. 2015;22:2082–7.
Metildi CA, Kaushal S, Luiken GA, Hoffman RM, Bouvet M. Advantages of fluorescence-guided laparoscopic surgery of pancreatic cancer labeled with fluorescent anti-carcinoembryonic antigen antibodies in an orthotopic mouse model. J Am Coll Surg. 2014;219:132–41.
Metildi CA, Kaushal S, Pu M, Messer KA, Luiken GA, Moossa AR, et al. Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer. Ann Surg Oncol. 2014;21:1405–11.
Metildi CA, Tang CM, Kaushal S, Messer KA, Luiken GA, Moossa AR, et al. In vivo fluorescence imaging of gastrointestinal stromal tumors using fluorophore-conjugated anti-KIT antibody. Ann Surg Oncol. 2013;20 Suppl 3:S693–700.
Tran Cao HS, Kaushal S, Metildi CA, Menen RS, Lee C, Snyder CS, et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology. 2012;59:1994–9.
van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–9.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
Koshi R, Mustafa Y, Perry ME. Vimentin, cytokeratin 8 and cytokeratin 18 are not specific markers for M-cells in human palatine tonsils. J Anat. 2001;199:663–74.
Wu Y, Wang X, Wu F, Huang R, Xue F, Liang G, et al. Transcriptome profiling of the cancer, adjacent non-tumor and distant normal tissues from a colorectal cancer patient by deep sequencing. PloS One. 2012;7:e41001.
Shahid MW, Buchner AM, Coron E, Woodward TA, Raimondo M, Dekker E, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest Endosc. 2012;75:525–33.
Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris Classification. Dig Dis Sci. 2013;58:1784–9.
Sharma P, Bansal A. Toward better imaging of Barrett’s esophagus—see more, biopsy less! Gastrointest Endosc. 2006;64:188–92.
Neumann H, Vieth M, Atreya R, Neurath MF, Mudter J. Prospective evaluation of the learning curve of confocal laser endomicroscopy in patients with IBD. Histol Histopathol. 2011;26:867–72.
Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13.
Schmidt C, Lautenschlager C, Petzold B, Sakr Y, Marx G, Stallmach A. Confocal laser endomicroscopy reliably detects sepsis-related and treatment-associated changes in intestinal mucosal microcirculation. Br J Anaesth. 2013;111:996–1003.
Becker V, Wallace MB, Fockens P, von Delius S, Woodward TA, Raimondo M, et al. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos). Gastrointest Endosc. 2010;71:1260–6.
Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, et al. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316–23.
Yanagita S, Natsugoe S, Uenosono Y, Arima H, Kozono T, Ehi K, et al. Morphological distribution of metastatic foci in sentinel lymph nodes with gastric cancer. Ann Surg Oncol. 2008;15:770–6.
Acknowledgments
Authors are grateful to Francine Gossé and Emilie Heuillard, laboratory technicians, for preparing the marked cells and to Mathilde Raux-Defossez, Christopher Burel, and Guy Temporal, professionals in medical English proofreading, for their valuable help in revising the manuscript.
Disclosure
Jacques Marescaux is the President of the IRCAD and IHU-Strasbourg Institutes, which are partly funded by Siemens Healthcare, Karl Storz Endoskope, and Covidien (Medtronic). Remaining authors have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4928_MOESM4_ESM.tif
CK18 staining/A-D) CK18 staining of liver (20x and 40x respectively). B-E) CK18 staining of the gastric mucosa at theinjection site (20x and 40x respectively). C-F) CK18 staining of a Negative node (20x and 40x respectively) (TIFF 13536 kb)
Rights and permissions
About this article
Cite this article
Diana, M., Robinet, E., Liu, YY. et al. Confocal Imaging and Tissue-Specific Fluorescent Probes for Real-Time In Vivo Immunohistochemistry. Proof of the Concept in a Gastric Lymph Node Metastasis Model. Ann Surg Oncol 23 (Suppl 5), 567–573 (2016). https://doi.org/10.1245/s10434-015-4928-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4928-y