Abstract
Background
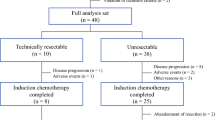
The Kyushu Study Group of Clinical Cancer (KSCC) conducted phase II trials of KSCC1002 (UMIN000001308) concerning liver resectability after first-line treatment of initially unresectable or not optimally resectable colorectal liver metastases in a prospective, multicenter study.
Methods
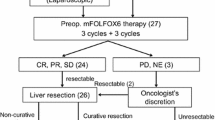
Patients with wild-type KRAS received 4–6 cycles of S-1 and oxaliplatin (SOX) plus cetuximab. Liver resectability was evaluated subsequently with the liver resection rate as the primary endpoint.
Results
Of the 33 patients enrolled between March 2010 and July 2013, the median number of administration cycles was 4 (range 0–10). The overall response rate was 63.6 % (95 % confidence interval [CI] 45.1–79.6 %). Liver resection was possible in 16 of 33 (48.5 %) patients, and there were 13 R0 cases (39.4 %). We conducted a central review of liver resectability evaluated by five liver surgeons, and the resectability increased from 18.2 to 66.7 % after chemotherapy, based on imaging. The median overall survival for all 33 cases was 31.6 months (95 % CI 14.8–not reached). The median progression-free survival was 9.7 months (95 % CI 6.2–11.8).
Conclusions
SOX plus cetuximab is safe and effective for advanced colorectal cancer with limited liver metastasis, and may lead to high liver resectability.



Similar content being viewed by others
References
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29.
Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg. 1989;210:127–38.
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–8.
Foster JH. Treatment of metastatic disease of the liver: a skeptic’s view. Semin Liver Dis. 1984;4:170–9.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Marino D, Leone F, D’Avanzo F, Ribero D, Capussotti L, Aglietta M. Potentially resectable metastatic colorectal cancer: an individualized approach to conversion therapy. Crit Rev Oncol Hematol. 2014;92:218–26.
Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25:1018–25.
Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:141–52; quiz 152.
Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385–90.
Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20:4289–97.
Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013;24:2062–7.
Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8.
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20.
Nakamura M, Yamada Y, Muro K, Takahashi K, Baba H, Sasaki Y, et al. The SOFT trial: a phase III study of the dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine S-1 and oxaliplatin (SOX) plus bevacizumab as first-line chemotherapy for metastatic colorectal cancer. Future Oncol. 2015;11:1471–8.
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278–86.
Kim ST, Hong YS, Lim HY, Lee J, Kim TW, Kim KP et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: updated results from a phase 3 trial. BMC Cancer. 2014;14:883.
Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a randomised, non-inferiority phase 3 trial. Lancet Oncol. 2012;13:1125–32.
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65.
Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700.
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47.
Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542–7.
Madi A, Fisher D, Wilson RH, Adams RA, Meade AM, Kenny SL, et al. Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial. Br J Cancer. 2012;107:1037–43.
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14.
Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12:642–53.
Adams RA, Meade AM, Madi A, Fisher D, Kay E, Kenny S, et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br J Cancer. 2009;100:251–8.
Beppu T, Emi Y, Tokunaga S, Oki E, Shirabe K, Ueno S, et al. Liver resectability of advanced liver-limited colorectal liver metastases following mFOLFOX6 with Bevacizumab (KSCC0802 Study). Anticancer Res. 2014;34:6655–62.
Gruenberger T, Bridgewater J, Chau I, Alfonso P, Rivoire M, Lasserre S, et al. Randomized, phase II study of bevacizumab with mFOLFOX6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: resectability and safety in OLIVIA. J Clin Oncol. 2013;31:3619.
Takahashi T, Shibata Y, Tojima Y, Tsuboi K, Sakamoto E, Kunieda K, et al. Multicenter phase II study of modified FOLFOX6 as neoadjuvant chemotherapy for patients with unresectable liver-only metastases from colorectal cancer in Japan: ROOF study. Int J Clin Oncol. 2013;18:335–42.
Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–51.
Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870–6.
Acknowledgment
The authors thank the participating patients and their families. We are indebted to the physicians and all other medical staff. We also thank Ms. Sakamoto and the staff at the Clinical Research Support Center Kyushu for their excellent data collection and management, secretarial assistance, and support.
Conflict of interest
No disclosures: YE, YMi, AK, HH, YO, MI, HS, ST, KS, MT, SU, MS and SN; College course financially maintained by private donations that Yakult Honsha Co. provides: TB and YMa; College course financially maintained by private donations that Taiho Pharmaceutical Co. provides: YMa; Acceptance such as the researchers from Taiho Pharmaceutical Co., Ltd: YMa; Grant research funding from Taiho Pharmaceutical Co., Ltd.: SE, YA, HB and YMa; Grant research funding from Yakult Honsha Co., Ltd.: SE, YA, HB; Lecturer’s fee from Taiho Pharmaceutical Co., Ltd.: YE, SE, YK, HB; Lecturer’s fee from Yakult Honsha Co., Ltd.: YE, SE, HB and YMa; Lecturer’s fee from Taiho Pharmaceutical Co., Ltd. and Yakult Honsha Co., Ltd.: YA.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4771_MOESM1_ESM.docx
Representative images before (A) and after (B) chemotherapy. A: Before chemotherapy.A 70-mm metastatic tumor contacts the hepatic vein. B: After chemotherapy. The tumorwas reduced to 28 mm and was separated from the vein. Supplementary material 1 (DOCX 15 kb)
10434_2015_4771_MOESM2_ESM.pdf
Cumulative progression-free survival (A) and overall survival (B) curves in patients withinitially unresectable liver metastases, according to the existence or nonexistence ofhepatic resection. Solid line: patients with hepatic resection, dotted line: patients withouthepatic resection. Supplementary material 2 (PDF 528 kb)
Rights and permissions
About this article
Cite this article
Oki, E., Emi, Y., Miyamoto, Y. et al. Phase II Trial of S-1 and Oxaliplatin Plus Cetuximab for Colorectal Cancer Patients with Initially Unresectable or Not Optimally Resectable Liver Metastases (KSCC1002). Ann Surg Oncol 22 (Suppl 3), 1067–1074 (2015). https://doi.org/10.1245/s10434-015-4771-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4771-1