Abstract
Background
Cancerous inhibitor of protein phosphatase 2A (CIP2A) is an oncoprotein inhibiting proteolytic degradation of c-MYC. In this study, we investigated the clinical relevance of CIP2A in NSCLC.
Materials and Methods
We analyzed CIP2A mRNA expression in 29 NSCLC tissues using quantitative reverse transcription polymerase chain reaction (RT-QPCR). We also examined the expression of CIP2A protein by immunohistochemistry in 90 lung cancer specimens and correlated its expression with c-MYC expression and clinicopathological parameters. The functional roles of CIP2A in lung cancer cell lines were evaluated by small interfering RNA-mediated depletion of the protein followed by analyses of cell proliferation and invasion.
Results
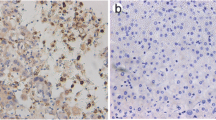
In 29 lung cancer tissues examined, 24 of them (82.7%) exhibited much stronger levels of CIP2A mRNA compared with their corresponding normal tissues. Moreover, CIP2A mRNA expression levels correlated with c-MYC mRNA levels. Furthermore, CIP2A protein was found to be overexpressed in 72.2% of 90 human lung cancer samples and correlated with poor survival (P < 0.05). In addition, the CIP2A status was a significant prognostic factor for NSCLC patients (P = 0.0136). Depleting CIP2A expression inhibited growth and clonogenic potential in lung cancer cell lines.
Conclusions
CIP2A is an oncoprotein overexpressed in NSCLC, and its expression is associated with poor prognosis and malignant cell proliferation.




Similar content being viewed by others
References
Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909.
O’Mahony D, Kummar S, Gutierrez ME. Non-small-cell lung cancer vaccine therapy: a concise review. J Clin Oncol. 2005;23:9022–8.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32.
Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44.
Zhang YA, Nemunaitis J, Samuel SK, Chen P, Shen Y, Tong AW. Antitumor activity of an oncolytic adenovirus-delivered oncogene small interfering RNA. Cancer Res. 2006;66:9736–43.
Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62.
Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101:793–805.
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia J, et al. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–8.
Côme C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–100.
Burke L, Flieder DB, Guinee DG, Brambilla E, Freedman AN, Bennet WP, et al. Prognostic implications of molecular and immunohistochemical profiles of the Rb and p53 cell cycle regulatory pathways in primary non-small cell lung carcinoma. Clin Cancer Res. 2005;11:232–41.
Arnold HK, Sears RC. A tumor suppressor role for PP2A-B56alpha through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev. 2008;27:147–58.
Junttila MR, Westermarck J. Mechanisms of MYC stabilization in human malignancies. Cell Cycle. 2008;7:592–6.
Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3:1133–7.
Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–18.
Dang CV, Resar LM, Emison E, Kim S, Li Q, Prescott JE, et al. Function of the c-Myc oncogenic transcription factor. Exp Cell Res. 1999;253:63–77.
Volm M, Koomagi R. Prognostic relevance of c-Myc and caspase-3 for patients with non-small cell lung cancer. Oncol Rep. 2000;7:95–8.
Yang G, Timme TL, Frolov A, Wheeler TM, Thompson TC. Combined c-Myc and caveolin-1 expression in human prostate carcinoma predicts prostate carcinoma progression. Cancer. 2005;103:1186–94.
Qian J, Hirasawa K, Bostwick DG, Bergstralh EJ, Slezak JM, Anderl KL, et al., Loss of p53 and c-myc overrepresentation in stage T(2–3)N(1–3)M(0) prostate cancer are potential markers for cancer progression. Mod Pathol. 2002;15:35–44.
Sharrard RM, Royds JA, Rogers S, Shorthouse AJ. Patterns of methylation of the c-myc gene in human colorectal cancer progression. Br J Cancer. 1992;65:667–72.
Watson PH, Safneck JR, Le K, Dubik D, Shiu RP. Relationship of c-myc amplification to progression of breast cancer from in situ to invasive tumor and lymph node metastasis. J Natl Cancer Inst. 1993;85:902–7.
Huang CL, Liu D, Ishikawa S, Nakashima T, Nakashima N, Yokomise H, et al. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer. 2008;44:2680–8.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (No. 30470764 and No. 30670917 to En-Hua Wang, No. 30670888 to Chen Zhao).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2010_1313_MOESM1_ESM.pdf
Supplement Figure 1 A Real-time PCR analyses of CIP2A depletion efficiency in HBE cells. B MTT assay was performed after CIP2A siRNA treatment in HBE cells. A slight reduction of absorbance was observed. C and D Assessment of clonogenic potentials of the CIP2A siRNA treated HBE cells. Number of colonies was counted. The number of colonies formed by cells treated with CIP2A siRNA (138 ± 23, mean ± SD) was comparable with that of control cells (165 ± 12, mean ± SD) (P = 0.159). (PDF 49 kb)
Rights and permissions
About this article
Cite this article
Dong, QZ., Wang, Y., Dong, XJ. et al. CIP2A is Overexpressed in Non-Small Cell Lung Cancer and Correlates with Poor Prognosis. Ann Surg Oncol 18, 857–865 (2011). https://doi.org/10.1245/s10434-010-1313-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1313-8