Abstract
Background
Histone deacetylases (HDACs) modulate chromatin and may influence the effect of DNA-damaging drugs. We investigated HDAC1 and -2 expression in gastric carcinomas (GCs) for an association of patient outcome with conventional neoadjuvant chemotherapy. In vitro, HDAC inhibitors were evaluated as alternative treatment options.
Methods
HDAC1/2 expression was analyzed immunohistochemically in 127 pretherapeutic biopsy samples of neoadjuvant (platinum/5-fluorouracil) chemotherapy-treated GC patients and correlated with response and overall survival (OS). Chemosensitivity of four GC cell lines to cisplatin and the HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) and valproic acid was determined by XTT assays. Efficiencies of combined drug schedules were analyzed.
Results
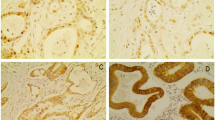
High expression of HDAC1/2 was found in 69 (54%) of 127 and 108 (85%) of 127 carcinomas, respectively, and was not associated with response or OS. In patients whose disease responded to therapy, high HDAC1 expression was associated with worse OS (P = 0.005). In cell lines, sequential treatment with SAHA and cisplatin showed synergistic effects irrespective of the initial cisplatin sensitivity.
Conclusions
HDAC1 and -2 expression is not suitable to predict response or survival for neoadjuvant-treated GC patients, but HDAC1 expression may be used for risk stratification in patients whose disease responds to therapy. Sequential treatment with SAHA and cisplatin may represent an alternative treatment option for GC patients.



Similar content being viewed by others
References
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Lordick F, Siewert JR. Recent advances in multimodal treatment for gastric cancer: a review. Gastric Cancer. 2005;8:78–85.
Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–32.
Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol. 2009;625:131–42.
Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92.
Napieralski R, Ott K, Kremer M, et al. Methylation of tumor-related genes in neoadjuvant-treated gastric cancer: relation to therapy response and clinicopathologic and molecular features. Clin Cancer Res. 2007;13:5095–102.
Kim MS, Blake M, Baek JH, et al. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–300.
Lin CT, Lai HC, Lee HY, et al. Valproic acid resensitizes cisplatin-resistant ovarian cancer cells. Cancer Sci. 2008;99:1218–26.
Davies NP, Hardman LC, Murray V. The effect of chromatin structure on cisplatin damage in intact human cells. Nucleic Acids Res. 2000;28:2954–8.
Weichert W, Roske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139–48.
Weichert W, Roske A, Niesporek S, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669–77.
Suzuki J, Chen YY, Scott GK, et al. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res. 2009;15:3163–71.
Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51.
Schuhmacher CP, Fink U, Becker K, et al. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001;91:918–27.
Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer. 2003;6:159–67.
Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–10.
Stocker G, Ott K, Henningsen N, et al. CyclinD1 and interleukin-1 receptor antagonist polymorphisms are associated with prognosis in neoadjuvant-treated gastric carcinoma. Eur J Cancer. 2009;45:3326–35.
Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–30.
Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55.
Gyorffy B, Surowiak P, Kiesslich O, et al. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. Int J Cancer. 2006;118:1699–712.
Kelly WK, Richon VM, O’Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88.
Fritzsche FR, Weichert W, Roske A, et al. Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer. BMC Cancer. 2008;8:381.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33.
Zhu P, Martin E, Mengwasser J, et al. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–63.
Lee JH, Park JH, Jung Y, et al. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol Cancer Ther. 2006;5:3085–95.
Owonikoko TK, Ramalingam SS, Kanterewicz B, et al. Vorinostat increases carboplatin and paclitaxel activity in non–small cell lung cancer cells. Int J Cancer. 126:743–55.
Zhang X, Yashiro M, Ren J, Hirakawa K. Histone deacetylase inhibitor, trichostatin A, increases the chemosensitivity of anticancer drugs in gastric cancer cell lines. Oncol Rep. 2006;16:563–8.
Gorisch SM, Wachsmuth M, Toth KF, et al. Histone acetylation increases chromatin accessibility. J Cell Sci. 2005;118:5825–34.
Dejligbjerg M, Grauslund M, Litman T, et al. Differential effects of class I isoform histone deacetylase depletion and enzymatic inhibition by belinostat or valproic acid in HeLa cells. Mol Cancer. 2008;7:70.
Acknowledgment
This work was supported by the Wilhelm-Sander-Stiftung (grant 2006.035-1 to G.K. and K.O.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(a) Expression of HDAC1 in cell pellets of MKN28, MKN45, AGS and KATOIII cells determined by immunohistochemistry is shown. Original magnification 1:400 (b) Western blot analysis of HDAC1 expression in cell lysates. β-actin expression served as loading control (c) Densitometric analysis of the HDAC1 expression normalised to β-actin expression levels
Supplementary material 2 (EPS 7591 kb)
Rights and permissions
About this article
Cite this article
Mutze, K., Langer, R., Becker, K. et al. Histone Deacetylase (HDAC) 1 and 2 Expression and Chemotherapy in Gastric Cancer. Ann Surg Oncol 17, 3336–3343 (2010). https://doi.org/10.1245/s10434-010-1182-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1182-1