Abstract
Background
Liver regeneration after hepatectomy stimulates metastatic tumor growth through the release of potent growth factors. In the signaling cascades of several growth factors, mTOR is a downstream mediator, triggering cell survival and cell cycle progression. The mTOR inhibitor rapamycin (RAPA) has been shown to exhibit potent antitumor activities. However, it is unknown whether RAPA is capable of exerting these effects under the conditions of hepatectomy-associated liver regeneration. We therefore analyzed the effect of RAPA and cyclosporine A (CyA) on tumor growth characteristics after major hepatectomy using a mouse model of colorectal metastasis.
Methods
Tumor growth was studied by using GFP-transfected CT26.WT colorectal cancer cells, which were implanted into the dorsal skinfold chambers of BALB/c-mice after 70% hepatectomy. The animals were treated daily with RAPA (1.5 mg/kg) or CyA (10 mg/kg). Tumors were analyzed for angiogenesis, microvascular blood perfusion, cell proliferation, apoptotic cell death, and tumor growth.
Results
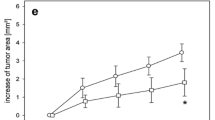
RAPA significantly inhibited tumor growth compared with CyA and sham treatment. This was associated with a decreased microvascular density within the tumors and a markedly reduced microvascular blood perfusion. CyA only slightly reduced angiogenesis and tumor growth. The effects of RAPA were associated with a significant reduction of tumor cell proliferation, whereas manifestation of apoptotic cell death was not affected by the immunosuppressive treatment regimen.
Conclusions
RAPA is capable of inhibiting angiogenesis, microvascular blood perfusion, and tumor growth of colorectal metastasis during hepatectomy-associated liver regeneration. Thus, targeting mTOR might represent an interesting strategy to prevent tumor recurrence after hepatectomy for colorectal metastasis.






Similar content being viewed by others
References
Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–27.
Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–22.
Oe H, Kaido T, Mori A, Onodera H, Imamura M. Hepatocyte growth factor as well as vascular endothelial growth factor gene induction effectively promotes liver regeneration after hepatectomy in Solt-Farber rats. Hepatogastroenterology. 2005;52:1393–7.
Yoon SS, Kim SH, Gonen M, et al. Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2006;13:353–62.
Panis Y, Ribeiro J, Chrétien Y, Nordlinger B. Dormant liver metastases: an experimental study. Br J Surg. 1992;79:221–3.
Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124:57–64.
Rupertus K, Kollmar O, Scheuer C, Junker B, Menger MD, Schilling MK. Major but not minor hepatectomy accelerates engraftment of extrahepatic tumor cells. Clin Exp Metastasis. 2007;24:39–48.
Slooter GD, Marquet RL, Jeekel J, Ijzermans JN. Tumour growth stimulation after partial hepatectomy can be reduced by treatment with tumour necrosis factor alpha. Br J Surg. 1995;82:129–32.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008; 371:1007–16.
Drixler TA, Borel Rinkes IH, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE. Continuous administration of angiostatin inhibits accelerated growth of colorectal liver metastases after partial hepatectomy. Cancer Res. 2000;60:1761–5.
Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist. 2008;13:139–47.
Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1–15.
Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35.
Ma WW, Hidalgo M. Exploiting novel molecular targets in gastrointestinal cancers. World J Gastroenterol. 2007;13:5845–56.
Palmes D, Zibert A, Budny T, et al. Impact of rapamycin on liver regeneration. Virchows Arch. 2008;452:545–57.
Kollmar O, Schilling MK, Menger MD. Experimental liver metastasis: standards for local cell implantation to study isolated tumor growth in mice. Clin Exp Metastasis. 2004;21:453–60.
Menger MD, Laschke MW, Vollmar B. Viewing the microcirculation through the window: some twenty years experience with the hamster dorsal skinfold chamber. Eur Surg Res. 2002;34:83–91.
Kollmar O, Rupertus K, Scheuer C, Junker B, Tilton B, Schilling MK, et al. Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis. Neoplasia. 2007;9:862–70.
Contaldo C, Meier C, Elsherbiny A, Harder Y, Trentz O, Menger MD, et al. Human recombinant erythropoietin protects the striated muscle microcirculation of the dorsal skinfold from postischemic injury in mice. Am J Physiol Heart Circ Physiol. 2007;293:H274–83.
Kollmar O, Corsten M, Scheuer C, Vollmar B, Schilling MK, Menger MD. Portal branch ligation induces a hepatic arterial buffer response, microvascular remodeling, normoxygenation, and cell proliferation in portal blood-deprived liver tissue. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1534–42.
Francavilla A, Starzl TE, Scotti C, et al. Inhibition of liver, kidney, and intestine regeneration by rapamycin. Transplantation. 1992;53:496–8.
Jiang YP, Ballou LM, Lin RZ. Rapamycin-insensitive regulation of 4e-BP1 in regenerating rat liver. J Biol Chem. 2001;276:10943–51.
Kirimlioglu H, Kirimlioglu V, Yilmaz S, Coban S, Turkmen E, Ara C. Liver pathology and cell proliferation after calcineurin inhibitors and antiproliferative drugs following partial hepatectomy in rats. Transplant Proc. 2006;38:622–6.
Francavilla A, Starzl TE, Barone M, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatology. 1991;14:140–3.
Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88.
Guba M, Yezhelyev M, Eichhorn ME, et al. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005;105:4463–9.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–62.
Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3.
Bruns CJ, Koehl GE, Guba M, et al. Rapamycin-induced endothelial cell death and tumor vessel thrombosis potentiate cytotoxic therapy against pancreatic cancer. Clin Cancer Res. 2004;10:2109–19.
Vajkoczy P, Vollmar B, Wolf B, Menger MD. Effects of cyclosporine A on the process of vascularization of freely transplanted islets of Langerhans. J Mol Med. 1999;77:111–4.
Wilasrusmee C, Yusupov I, Ondocin P, Bruch D, Kittur S, Wilasrusmee S, et al. Angiocidal effect of Cyclosporin A: a new therapeutic approach for pathogenic angiogenesis. Int Angiol. 2005; 24:372–9.
Shah G, Middleton FA, Gentile KL, Tripathi S, Bruch D, Maier KG, et al. Cyclosporine inhibition of angiogenesis involves the transcription factor HESR1. J Surg Res. 2008;149:171–6.
Rad FH, Le Buanec H, Paturance S, et al. VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc Natl Acad Sci USA. 2007;104:2837–42.
Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–55.
Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immuno-suppression? Langenbecks Arch Surg. 2004;389:475–84.
Acknowledgement
The authors appreciate the excellent technical assistance of Christina Marx, Janine Becker, and Claudia Scheuer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rupertus, K., Dahlem, C., Menger, M.D. et al. Rapamycin Inhibits Hepatectomy-Induced Stimulation of Metastatic Tumor Growth by Reduction of Angiogenesis, Microvascular Blood Perfusion, and Tumor Cell Proliferation. Ann Surg Oncol 16, 2629–2637 (2009). https://doi.org/10.1245/s10434-009-0564-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0564-8