Abstract
The receptor for advanced glycation end products (RAGE), known as a multiligand receptor for certain stress-associated factors, has been considered to affect the characteristic differences of various cancer cells. We analyzed the expression and clinicopathological significance of RAGE in esophageal squamous cell carcinoma. We investigated immunohistochemically the relationship between RAGE expression and clinicopathological factors, including prognosis, in surgical specimens of primary tumors in 216 patients with esophageal squamous cell carcinoma. Prognostic factors were examined by univariate and multivariate analyses (Cox proportional hazard regression model). The positive expression rate of RAGE was 50%. RAGE expression was negatively correlated with depth of invasion and venous invasion. Moreover, tumors with positive RAGE expression exhibited better prognosis than those with negative RAGE expression (5-year survival, 52% vs. 32%, respectively). Multivariate analysis indicated that the positive expression of RAGE was an independent prognostic factor, along with tumor depth and nodal metastasis. Our findings suggest that loss of RAGE expression may play an important role in the progression of esophageal squamous cell carcinoma. Evaluation of the expression of RAGE could be useful for determining the tumor properties, including those associated with prognosis, in patients with esophageal squamous cell carcinoma.
Similar content being viewed by others
The receptor for advanced glycation end products (RAGE) is a multiligand receptor classified as an immunoglobulin superfamily cell surface molecule; it acts as a counterreceptor for high-mobility group box 1 (HMGB1) proteins, RAGEs, S100/calgranulins, and amyloid-β peptides.1–3 These interactions trigger the activation of key cell signaling pathways (e.g., p38 and p44/42 MAP kinase, NF-κB, cdc42/rac, and the generation of reactive oxygen species) and result in the production of proinflammatory cytokines.4–7 RAGE-mediated proinflammatory processes are now considered to contribute to the progression of many chronic diseases, such as neuropathy, nephropathy, macrovascular disease, amyloidoses, inflammatory conditions (e.g., rheumatoid arthritis and inflammatory bowel disease), and sepsis.7–9 In addition to RAGE-mediated proinflammatory events, recent studies have revealed that the interaction of RAGE and its ligands and the resultant signaling play a causative role in the characteristic modulation of cancer cell functions, i.e., increasing tumor invasion and metastasis.10 Furthermore, several clinical studies have demonstrated a strong association of RAGE expression with the malignant potential of various cancers such as gastric cancer, colon cancer, biliary cancer, pancreatic cancer, and prostate cancer, although several reports showed a reverse correlation between RAGE expression and tumor progression.11–19
We previously reported a change in RAGE expression according to the sequential change of liver tissue.20 The RAGE expression was higher in the early or prestages of cancer than in the normal liver or advanced hepatocellular carcinoma (HCC). An HCC lesion, which is highly associated with inflammation caused by either hepatitis viruses or drugs, develops through a process of multistage carcinogenesis, i.e., from inflammatory lesions (e.g., hepatitis, cirrhosis and precancerous lesion) to dysplastic nodules, and eventually into HCC.
Esophagus squamous cell carcinoma (ESCC) is one of the most aggressive carcinomas of the gastrointestinal tract. Many studies have identified various biological factors in the malignant potential of ESCC. ESCC is also thought to develop through a process of multistage carcinogenesis. Most ESCC arises from esophageal dysplasia caused by smoking and drinking. Thus, RAGE expression may be expected to play an important role in the development of ESCC, although no data have been reported for this tumor. In this study, we intended to examine the expression of RAGE in surgical specimens of ESCC and to provide some indication of the biological role of RAGE in ESCC.
Materials and Methods
Patients and Tumor Specimens
The present study involved 216 consecutive patients with thoracic ESCC (199 men and 17 women) who underwent curative surgery at the Kagoshima University Hospital between January 1987 and December 1998. All of these patients underwent an esophagectomy with lymph node dissection. The cases of operation-related death within 30 days after the esophagectomy were excluded. The patients ranged in age from 38 to 84 years (mean, 64.1 years). None of these patients underwent endoscopic mucosal resection, palliative resection, preoperative chemotherapy, or radiotherapy, and none of them had synchronous or metachronous multiple cancers in other organs. Of 216 patients, 117 (54.1%) died of relapse of ESCC and were categorized as uncensored patients. Among these patients, 54 experienced lymph node recurrences, 50 distant organ recurrence, and 29 local recurrence. Data from patients who died of disease other than ESCC or its recurrences were censored.
Specimens of cancer and adjacent noncancerous tissues were collected from the patients according to the institutional guidelines of our hospital after informed consent had been obtained. Classifications of the specimens were determined according to the International Union Against Cancer tumor-node-metastasis classification system.21 All of the M1 tumors had distant lymph node metastases. All patients were followed up after discharge with a chest X-ray every 1–3 months, computed tomography every 3 to 6 months, and ultrasound every 6 months. Follow-up data after surgery were available for all patients, with a median follow-up period of 20 months (range, 2–166 months).
Immunohistochemical Staining and Evaluation
Tumor samples were fixed with 10% formaldehyde in phosphate-buffered saline (PBS), embedded in paraffin, and sectioned into 4-μm-thick slices. Immunohistochemical staining was performed by the conventional immunoperoxidase technique. After paraffin was removed from the sections, the endogenous peroxidase was blocked by immersing the slides in a 3% H2O2-methanol solution for 10 min at room temperature. As preparation for staining with RAGE antibodies, sections were treated with 1 mM EDTA-NaOH solution (pH 8.0) for 12 minutes at 100°C in a microwave oven.22 The sections were washed three times with PBS, each for 5 minutes, and then blocked by treatment with PBS containing 1.5% normal rabbit serum (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. The blocked sections were incubated with primary antibody RAGE (C-20, Santa Cruz Biotechnology, Santa Cruz, CA; 1:50), diluted in PBS for 2 h at room temperature, and then incubated with the secondary antibody conjugated with peroxidase at room temperature. The sections were washed in PBS for 5 min three times, and the immune complex was visualized by incubating the sections with diaminobenzidine tetrahydrochloride. The sections were rinsed briefly in water, counterstained with Meyer’s hematoxylin (Sigma Chemical Co., St. Louis, MO), and mounted. Negative controls were performed by replacing the primary antibodies with PBS. Evaluation of immunohistochemistry results was independently carried out by two surgeons (T.T. and S.N.) who had training in digestive pathology. To evaluate the expressions of RAGE, 10 fields (within the tumor and at the invasive front) were selected, and the expression in 1,000 tumor cells (100 per field) was evaluated with high-power (×200) microscopy. The immunohistochemical expression of RAGE was defined as positive if distinct staining of cell membrane or cytoplasm was observed in at least 10% of tumor cells.
Statistical Analysis
Statistical analysis of group differences was performed by the χ 2 test and t-test. The Kaplan–Meier method was used for survival analysis and differences in survival were estimated by the log-rank test. Prognostic factors were examined by univariate and multivariate analyses (Cox proportional hazard regression model). P < .05 was considered to be statistically significant. The P values in this study were two sided. All statistical analyses were performed by StatView software, version 5.0 (Abacus Concepts, Berkeley, CA).
Results
Expressions of RAGE in ESCC
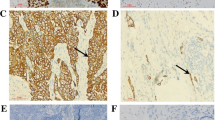
Positive and negative expression of RAGE is shown in Fig. 1. Information for eight patients (3.7% of total) were discordant among the evaluation of the specimens by the two investigators. Such specimens were omitted from this study. RAGE immunoreactivity was observed in the cell membrane and cytoplasm of tumor cells, but not in the nuclei. The RAGE-positive tumor cells frequently formed a cluster, but the RAGE-negative tumor cells did not (Fig. 1). Positive RAGE expression was found in 104 (50%) of 208 patients. Nevertheless, in the cases with tumors with negative RAGE expression, RAGE positive tumor cells were frequently found in a superficial site (Fig. 2a) rather than at the invasive front of the tumor (Fig. 2b). Weak to moderate RAGE immunoreactivity was observed in basal cells of noncancerous esophageal epithelium (Fig. 3).
Receptor for advanced glycation end products (RAGE) expression in esophageal squamous cell carcinoma (ESCC). a, b Positive RAGE expression (original magnification, ×200). Almost tumor cells show moderate to strong immunoreactivity (arrows). c, d Negative RAGE expression (original magnification, ×200). Almost tumor cells show no or weak immunoreactivity
Relationship Between the Expression of RAGE and Clinicopathological Findings
The expression of RAGE was significantly correlated with depth of tumor invasion and venous invasion. The tumors with negative RAGE expression invaded deeper and showed more venous invasion than the tumors with positive RAGE expression (P = .008 and P = .031, respectively). Age, sex, tumor location, histology of tumor, lymph node metastasis, tumor, node, metastasis stage, and lymphatic invasion were not correlated with the expression of RAGE (Table 1).
Relationship Between Prognosis and Expression of RAGE
The 5-year survival rate of patients with tumors with positive RAGE expression was 52.3%, whereas the rate for those with negative RAGE expression was 31.8%. There was a statistically significant difference in overall survival rates between patients with positive and negative expression of RAGE (Fig. 4). In disease-free survival, there was also a significant difference between RAGE-positive and -negative groups (P = .0020, 3-year disease-free survival rates were 54.8 and 33.7%, respectively).
Uni- and Multivariate Analysis of Prognostic Factors in ESCC
Univariate analysis showed that the following factors were significantly related to postoperative survival: histology, tumor depth, lymph node metastasis, stage, lymphatic invasion, venous invasion, and RAGE expression (P < .05) (Table 2). Multivariate regression analysis indicated that depth of invasion, lymph node metastasis, and RAGE expression were independent prognostic factors (Table 3).
Discussion
In the present study, we immunohistochemically investigated the relationship between RAGE expression and clinicopathological factors, including prognosis in ESCC. Our results show that negative RAGE expression correlates with deeper tumor invasion and venous invasion. Our data are consistent with observations in non–small lung carcinoma cells, in which downregulation of RAGE signaling was associated with tumor stage and with previous work showing that RAGE-induced neuronal differentiation was associated with proliferation arrest.15,23 Moreover, one study reported that RAGE expression in rhabdomyosarcoma reduced proliferation, migration, invasiveness and tumor growth, and functional inactivation of RAGE in L6 myoblasts resulted in increased proliferation and tumor formation in vivo.19,24 These data are also consistent with our results and suggest that a decrease in RAGE signaling is associated with tumor progression. However, our observations are at variance with the data obtained with glioma, gastric cancer, colon cancer, biliary cancer, and human pancreatic carcinoma cells, in which a positive correlation was found between RAGE expression, tumor progression, and metastasis.10–15 One study reported that RAGE expression positively correlates with the degree of atypia in colorectal adenomas because 90% (18 of 20) of adenomas with severe atypia express RAGE.25 However, in another study, investigators reported that RAGE positivity in cases with Dukes’ B, C, and D stage colorectal cancer was 19, 81, and 100%, respectively.13 There was a conflict between their results in colorectal adenoma and those in colorectal cancer according to the adenoma-adenocarcinoma sequence theory. Some of these conflicts may have a basis. RAGE interacts with several extracellular ligands that can be produced by the cell or by neighboring/circulating cells. Therefore, the tumor cells influence each other. Moreover, most RAGE ligands also fulfill intracellular functions independent of RAGE.
Another aspect of the biology of RAGE was suggested by the features of retinoic acid–induced neuroblastoma differentiation in which RAGE expression played a more important role in cellular survival than neurite outgrowth.26 In this model, inhibition of RAGE function partially blocked the increase in levels of the antiapoptotic protein Bcl-2 during the process of neuronal differentiation, indicating that RAGE and its signaling also might contribute to the survival of certain cancer cell types undergoing “differentiation.” We previously reported that increased RAGE expression was highly associated with the status of “differentiation” in HCC, which played an important role in acquisition of the hypoxia-resistant phenotype of tumor cells.20 Some recent studies revealed the differentiation-promoting activity of HMGB1/RAGE in neurons and L6 myoblasts.5,27 These studies’ findings are consistent with our results in HCC and emphasize that RAGE may play an important role in cellular differentiation. According to our current detailed observations, RAGE was highly expressed in noncancerous basal cells of esophageal epithelium. Basal cells of esophageal epithelium are actively differentiating into squamous epithelial cells. This observation supports the hypothesis that RAGE plays some key roles in cellular differentiation. Moreover, one study reported a positive correlation between RAGE immunoreactivity and histologic differentiation in oral squamous cell carcinoma.18 However, in the present study, RAGE expression in ESCC showed no marked correlation with “pathological” differentiation. Our previous report revealed that there was no marked correlation between the status of pathological differentiation and the expression of E-cadherin, which is one of the major intercellular adhesion molecules.28,29 The phenotype of E-cadherin expression might be recognized as one of the indicators of cellular differentiation in the epithelial cells. Therefore, pathological differentiation may not always reflect the cellular differentiation in the ESCC.
Our results show that negative RAGE expression correlates not only with deeper tumor invasion but also with the venous invasion. Because the esophageal wall and veins are common smooth muscle structures, the loss of RAGE expression may promote smooth muscle invasion by ESCC. We thought that some change in the microenviroment surrounding tumor cells may occur when the tumor cells invade smooth muscle tissue. Changes in the microenviroment may change the RAGE signaling and influence tumor invasion. As these results show, it seems that negative RAGE expression became an independent prognostic factor similar to depth of tumor invasion and lymph node metastasis. Various proteins have been considered as prognostic factors of ESCC. RAGE is an interesting molecule not only as a prognostic factor but also in regard to carcinogenesis or oncogenesis.
In conclusion, RAGE expression was negatively associated with depth of invasion, venous invasion, and prognosis. Our findings have provided the first evidence of the clinical relevance and function of RAGE in ESCC. Although other mechanisms may also be important, our data indicate that RAGE and its functions may be possible candidates for therapeutic targets in the treatment of ESCC. In addition, because RAGE expression was an independent prognostic factor, evaluation of RAGE expression may be useful for predicting malignant properties of ESCC.
References
Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262:16625–35.
Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901.
Du Yan S, Zhu H, Fu J, et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–301.
Yeh CH, Sturgis L, Haidacher J, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–504.
Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–24.
Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94.
Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51.
Yamamoto Y, Kato I, Doi T, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–8.
Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–50.
Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60.
Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–70.
Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer. 2003;104:722–7.
Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10:445–8.
Hirata K, Takada M, Suzuki Y, et al. Expression of receptor for advanced glycation end products (RAGE) in human biliary cancer cells. Hepatogastroenterology. 2003;50:1205–7.
Takada M, Koizumi T, Toyama H, et al. Differential expression of RAGE in human pancreatic carcinoma cells. Hepatogastroenterology. 2001;48:1577–8.
Ishiguro H, Nakaigawa N, Miyoshi Y, et al. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100.
Bartling B, Hofmann HS, Weigle B, et al. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non–small cell lung carcinoma. Carcinogenesis. 2005;26:293–301.
Landesberg R, Woo V, Huang L, et al. The expression of the receptor for glycation endproducts (RAGE) in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:617–24.
Riuzzi F, Sorci G, Donato R. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am J Pathol. 2007;171:947–61.
Hiwatashi K, Ueno S, Abeyama K, et al. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol. 2008;15:923–33.
Sobin LH, Fleming ID. TNM classification of malignant tumors, 5th ed. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–4.
Pileri SA, Roncador G, Ceccarelli C, et al. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997;183:116–23.
Huttunen HJ, Fages C, Kuja-Panula J, et al. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–11.
Riuzzi F, Sorci G, Donato R. The amphoterin (HMGB1)/receptor for advanced glycation end products (RAGE) pair modulates myoblast proliferation, apoptosis, adhesiveness, migration, and invasiveness. Functional inactivation of RAGE in L6 myoblasts results in tumor formation in vivo. J Biol Chem. 2006;281:8242–53.
Sasahira T, Akama Y, Fujii K, et al. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446:411–5.
Sajithlal G, Huttunen H, Rauvala H, et al. Receptor for advanced glycation end products plays a more important role in cellular survival than in neurite outgrowth during retinoic acid-induced differentiation of neuroblastoma cells. J Biol Chem. 2002;277:6888–97.
Sorci G, Riuzzi F, Arcuri C, et al. Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol Cell Biol. 2004;24:4880–94.
Uchikado Y, Natsugoe S, Okumura H, et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1174–80.
Nagafuchi A, Shirayoshi Y, Okazaki K, et al. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–3.
Acknowledgment
This work was supported by a Grants-in-Aid for Scientific Research from the Japan Society for the promotion of Science (no. 19591595 to SU, and no. 19591549 to SN).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tateno, T., Ueno, S., Hiwatashi, K. et al. Expression of Receptor for Advanced Glycation End Products (RAGE) is Related to Prognosis in Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 16, 440–446 (2009). https://doi.org/10.1245/s10434-008-0237-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-0237-z