Abstract
Purpose
We sought to determine whether smartphone GPS data uncovered differences in recovery after breast-conserving surgery (BCS) and mastectomy, and how these data aligned with self-reported quality of life (QoL).
Methods
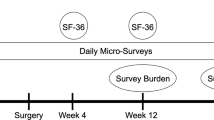
In a prospective pilot study, adult smartphone-owners undergoing breast surgery downloaded an application that continuously collected smartphone GPS data for 1 week preoperatively and 6 months postoperatively. QoL was assessed with the Short-Form-36 (SF36) via smartphone delivery preoperatively and 4 and 12 weeks postoperatively. Endpoints were trends in daily GPS-derived distance traveled and home time, as well as SF36 Physical (PCS) and Mental Component Scores (MCS) comparing BCS and mastectomy patients.
Results
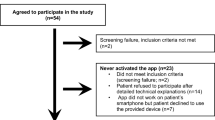
Thirty-one patients were included. Sixteen BCS and fifteen mastectomy patients were followed for a mean of 201 (SD 161) and 174 (107) days, respectively. There were no baseline differences in demographics, PCS/MCS, home time, or distance traveled. Through 12 weeks postoperatively, mastectomy patients spent more time at home [e.g., week 4: 16.7 h 95% CI (14.3, 19.6) vs. 11.0 h (9.4, 12.9), p < 0.001] and traveled shorter distances [e.g., week 4: 52.5 km 95% CI (36.1, 76.0) vs. 107.7 km (75.8–152.9), p = 0.009] compared with BCS patients. There were no significant QoL differences throughout the study as measured by the MCS [e.g., week 4 difference: 7.83 95% CI (− 9.02, 24.7), p = 0.362] or PCS [e.g., week 4 difference: 8.14 (− 6.67, 22.9), p = 0.281]. GPS and QoL trends were uncorrelated (ρ < ± 0.26, p > 0.05).
Conclusions
Differences in BCS and mastectomy recovery were successfully captured using smartphone GPS data. These data may describe currently unmeasured aspects of physical and mental recovery, which could supplement traditional and QoL outcomes to inform shared decision-making.




Similar content being viewed by others
References
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. https://doi.org/10.1056/nejmoa020989.
Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol (Madr). 2008;47(4):672–681. https://doi.org/10.1080/02841860801971439.
Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. https://doi.org/10.1016/s1470-2045(12)70042-6.
Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status. Cancer. 2000;89(11):2176–2186. https://doi.org/10.1002/1097-0142(20001201)89:11 %3c 2176::aid-cncr5 %3e 3.0.co;2-6.
Lee ES, Lee MK, Kim SH, et al. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg. 2011;253(1):101–108. https://doi.org/10.1097/sla.0b013e3181f662ce.
Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27(6):965–974. doi:10.1093/annonc/mdw099.
Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27(1):32. https://doi.org/10.1186/1756-9966-27-32.
Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214(1):1–10. https://doi.org/10.1016/j.jamcollsurg.2011.09.017.
Oskay-Ozcelik G, Lehmacher W, Konsgen D, et al. Breast cancer patients’ expectations in respect of the physician–patient relationship and treatment management results of a survey of 617 patients. Ann Oncol. 2006;18(3):479–484. https://doi.org/10.1093/annonc/mdl456.
Onnela J-P, Rauch SL. Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology. 2016;41(7):1691–1696. https://doi.org/10.1038/npp.2016.7.
Panda N, Solsky I, Haynes AB. Redefining shared decision-making in the digital era. Eur J Surg Oncol. 2019. https://doi.org/10.1016/j.ejso.2019.07.025.
Torous J, Kiang MV, Lorme J, Onnela J-P. New tools for new research in psychiatry: a scalable and customizable platform to empower data driven smartphone research. JMIR Ment Health. 2016;3(2):e16. https://doi.org/10.2196/mental.5165.
Panda N, Solsky I, Huang EJ, et al. Passively collected smartphone sensor data to detect postoperative events after cancer surgery: a prospective, multicenter, proof-of-principle study. J Am Coll Surg. 2019;229(4):S159–S160. https://doi.org/10.1016/j.jamcollsurg.2019.08.352.
Panda N, Solsky I, Huang EJ, et al. Using smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg. 2019. https://doi.org/10.1001/jamasurg.2019.4702.
Armstrong KA, Coyte PC, Brown M, Beber B, Semple JL. Effect of home monitoring via mobile app on the number of in-person visits following ambulatory surgery. JAMA Surg. 2017;152(7):622. https://doi.org/10.1001/jamasurg.2017.0111.
Hyder JA, Hirschberg RE, Nguyen LL. Home discharge as a performance metric for surgery. JAMA Surg. 2015;150(2):96. https://doi.org/10.1001/jamasurg.2014.1725.
Myles PS, Shulman MA, Heritier S, et al. Validation of days at home as an outcome measure after surgery: a prospective cohort study in Australia. BMJ Open. 2017;7(8):e015828. https://doi.org/10.1136/bmjopen-2017-015828.
Yurkiewicz IR, Simon P, Liedtke M, Dahl G, Dunn T. Effect of fitbit and iPad wearable technology in health-related quality of life in adolescent and young adult cancer patients. J Adolesc Young Adult Oncol. 2018;7(5):579–583. https://doi.org/10.1089/jayao.2018.0022.
Petersen J, Austin D, Kaye JA, Pavel M, Hayes TL. Unobtrusive in-home detection of time spent out-of-home with applications to loneliness and physical activity. IEEE J Biomed Health Inf. 2014;18(5):1590. https://doi.org/10.1109/jbhi.2013.2294276.
Panda N, Haynes AB. Prioritizing the patient perspective in oncologic surgery. Ann Surg Oncol. 2019. https://doi.org/10.1245/s10434-019-07753-6.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
Barnett I, Onnela J-P. Inferring mobility measures from GPS traces with missing data. Biostatistics. 2018. https://doi.org/10.1093/biostatistics/kxy059.
Panda N, Rattner DW, Morse CR. Third-time (“redo-redo”) anti-reflux surgery: patient-reported outcomes after a thoracoabdominal approach. Surg Endosc. 2019. https://doi.org/10.1007/s00464-019-07059-4.
RAND. 36-Item Short Form Survey (SF-36). https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. Accessed 14 Oct 2019.
Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JPA. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ. 2009;338:a3006. https://doi.org/10.1136/bmj.a3006.
Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. https://doi.org/10.1023/a:1012552211996.
Laucis NC, Hays RD, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Jt Surg Am Vol. 2014;97(19):1628–1634. https://doi.org/10.2106/jbjs.o.00030.
Mehta CR, Patel NR, Tsiatis AA. Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics. 1984;40(3):819. https://doi.org/10.2307/2530927.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. https://doi.org/10.1109/tac.1974.1100705.
Multiple comparisons—Handbook of Biological Statistics. http://www.biostathandbook.com/multiplecomparisons.html. Accessed 20 Feb 2020.
Petersen J, Austin D, Mattek N, Kaye J. Time Out-of-Home and Cognitive, physical, and emotional wellbeing of older adults: a longitudinal mixed effects model. PLoS One. 2015;10(10):e0139643. https://doi.org/10.1371/journal.pone.0139643.
Wettstein M, Wahl H-W, Shoval N, et al. Out-of-home behavior and cognitive impairment in older adults. J Appl Gerontol. 2015;34(1):3–25. https://doi.org/10.1177/0733464812459373.
Bade BC, Brooks MC, Nietert SB, et al. Assessing the correlation between physical activity and quality of life in advanced lung cancer. Integr Cancer Ther. 2018;17(1):73–79. https://doi.org/10.1177/1534735416684016.
de Mik SML, Stubenrouch FE, Balm R, Ubbink DT. Systematic review of shared decision-making in surgery. Br J Surg. 2018;105(13):1721–1730. https://doi.org/10.1002/bjs.11009.
Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. https://doi.org/10.1200/jco.1997.15.3.974.
FACT-B—Functional Assessment of Cancer Therapy—Breast Cancer. https://eprovide.mapi-trust.org/instruments/functional-assessment-of-cancer-therapy-breast-cancer. Accessed 15 Oct 2019.
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the breast-Q. Plast Reconstr Surg. 2009;124(2):345–353. https://doi.org/10.1097/prs.0b013e3181aee807.
Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer—taking stock. JNCI J Natl Cancer Inst. 2003;95(4):263–281. https://doi.org/10.1093/jnci/95.4.263.
Antonescu I, Scott S, Tran TT, Mayo NE, Feldman LS. Measuring postoperative recovery: what are clinically meaningful differences? Surgery. 2014;156(2):319–327. https://doi.org/10.1016/j.surg.2014.03.005.
Smith A. U.S. Smartphone Use in 2015| Pew Research Center.
Acknowledgments
This study was supported by a National Institutes of Health Research Training in Alimentary Tract Surgery grant (Grant T32 DK007754-18 [NP]), an Ariadne Labs Spark Grant funded by the Paul G. Allen Family foundation (NP, IS, JPO, ABH), and the National Institutes of Health/National Institute of Mental Health [Grant 1DP2MH103909 (JPO)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
NP reports a contract agreement with Aptima, a human-centered engineering and performance assessment contractor of the DARPA/Department of Defense. JPO receives sole compensation as a member of Harvard University. His research at Harvard T.H. Chan School of Public Health is supported by research awards from the NIH, Otsuka Pharmaceutical, Boehringer Ingelheim, and Apple. He received an unrestricted gift from Mindstrong Health in 2018. He is cofounder and board member of a recently established commercial entity that operates in digital phenotyping.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: GPS Data Sampling and Procedure Used to Process Raw GPS Data for Computing Daily Home Time and Distance Traveled
Appendix: GPS Data Sampling and Procedure Used to Process Raw GPS Data for Computing Daily Home Time and Distance Traveled
GPS data were sampled from smartphones by alternating between on-cycles (GPS data sampled) and off-cycles (no GPS data sampled). In this study the duration of the on-cycle was set to 2 min and the duration of the off-cycle to 10 min. This simple sampling scheme enables collection of enough data to infer mobility patterns while minimizing smartphone battery drainage.22 Importantly, this approach also enables quantification of the extent of missingness in data that is not due to design, but due to behavioral or other factors, such as patients turning off their phones. Patients were encouraged to use and interact with their phones as they normally would for the duration of the study.
GPS data were processed to compute daily “home time” (in hours) and “distance traveled” (in kilometers) metrics.22 Raw GPS data were collected at specific intervals according to a data collection schedule specified by the study design. Each sample recorded subjects’ latitude and longitude that were converted into a mobility trace defined by a sequence of flights and pauses. Flights were defined to be segments of linear movement and pauses were defined to be periods of time when a person did not move. Curved movement was approximated by multiple sequential flights. Also, if a missing interval was flanked by two pauses at the same location (situated within 50 m of one another), the missing interval was assumed to be a longer pause at the same location. Because the data stream has long periods of structured missingness, we next used a resampling method to estimate a complete connected path of flights and pauses. Specifically, each missing period was filled with random draws from the subjects’ empirical distributions of observed flights and pauses to create complete paths that reflected individuals’ observed mobility patterns. The resulting trajectories were summarized into fifteen daily summary statistics or metrics. Given the aims of this study, we selected two specific metrics that potentially describe aspects of postoperative recovery. The “distance traveled” metric is computed as the sum of the lengths (in kilometers) of the flights in each day (irrespective of mode of transportation). To compute “home time,” we first fixed the location of each subject’s home by identifying the set of significant locations the subject visited. In order to determine the set of all significant locations for a person, we ran a ‘k-means’ clustering algorithm on the set of all pause locations with a minimum duration of 10 min to identify a small number of locations where the subject spent the most time. The significant location with the largest total amount of time during the night hours between 9 p.m. and 6 a.m. over the course of the study period was assumed to be the location of the person’s home. The “home time” metric was the amount of time (in hours) per day spent within a 200-m radius of home.
Rights and permissions
About this article
Cite this article
Panda, N., Solsky, I., Hawrusik, B. et al. Smartphone Global Positioning System (GPS) Data Enhances Recovery Assessment After Breast Cancer Surgery. Ann Surg Oncol 28, 985–994 (2021). https://doi.org/10.1245/s10434-020-09004-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09004-5