Abstract
Background
Neoadjuvant chemotherapy (NAC) is known to downstage disease in the breast and increase breast conservation. It can also decrease nodal disease extent. We evaluated the impact of NAC on nodal positivity, nodal burden, and nodal surgery by tumor subtype.
Methods
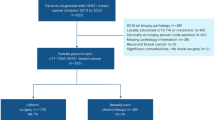
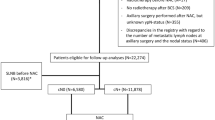
All cT1–4c breast cancers from 2010 to 2014 in the National Cancer Database were evaluated, comparing patients receiving NAC with those undergoing primary surgery (PS). Rates of pathologic node-negative status (pN0) and sentinel lymph node (SLN) surgery (1–5 nodes) were compared using chi-square tests, and adjusted odds ratios (OR) were estimated.
Results
Of 461,549 patients, 36,715 (8.0%) received NAC and 424,834 (92.0%) had PS. In cN0 patients, pN0 rates were higher in NAC compared with PS patients in ER−/HER2+ [93.2 vs. 79.0%, odds ratio (OR) 3.64, p < 0.001], ER−/HER2− (89.9 vs. 85.2%, OR 1.55, p < 0.001), and ER+/HER2+ (84.7 vs. 78.3%, OR 1.54, p < 0.001). Patients with cT2–3, N0 tumors had significantly higher rate of SLN surgery for NAC versus PS for each biologic subtype except for ER+/HER2− tumors, amongst which this was true only for T3 tumors. In cN1–3 patients, pN0 rates after NAC were 61.3% in ER−/HER2+, 47.7% in ER+/HER2+, 47.3% in ER−/HER2−, and 20.2% in ER+/HER2− and SLN surgery was highest in ER−/HER2+ (28.9%, p < 0.05 versus other subtypes).
Conclusion
NAC increases rates of pN0 among cN0 patients compared with PS. Among cN+ patients, 20–61% undergoing NAC convert to pN0 depending on tumor type, with lowest nodal response in ER+/HER2− disease. Use of NAC results in less extensive axillary surgery than in patients treated with PS in both cN0 and cN+ disease.

Similar content being viewed by others
References
NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer [Internet]. Version 2.2017. www.nccn.org/patients. 2017. p. 1–201.
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;15212(30):96–102.
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001 Nov 15;19(22):4224–37.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608-14-6.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013 Oct 9;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013 Jun;14(7):609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015 Jan 20;33(3):258–64.
AJCC (American Joint Committee on Cancer) (2010) Breast cancer staging, Cancer staging manual, 7th edition. Springer, New York
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384(9938):164–72.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012 May 20;30(15):1796–804.
Mougalian SS, Hernandez M, Lei X, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016 Apr;2(4):508-16.
American Joint Committee on Cancer (AJCC) (2017) AJCC Cancer Staging Manual. 8th ed. Springer, New York
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633-40.
Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84.
Untch M, Loibl S, Bischoff J, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–44.
Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884–9.
Loibl S, von Mickwitz G, Blohmer J, Costa S, Eidtmann H, Fasching P, et al. pCR as a surrogate in HER2-positive patients treated with trastuzumab. Cancer Res. 2011;71(24 Suppl): Abstract S5-4
Valero V. Carboplatin for early triple-negative breast cancer? Lancet Oncol. 2014;15(7):676–8.
Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–5.
Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–9.
von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56.
Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21.
Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12(11):1527–32.
Ellis MJ, Coop A, Singh B, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;63(19):6523–31.
Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor–rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011 Jun 10;29(17):2342–9.
Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106(10):2095–103.
Seo JH, Kim YH, Kim JS. Meta-analysis of pre-operative aromatase inhibitor versus tamoxifen in postmenopausal woman with hormone receptor-positive breast cancer. Cancer Chemother Pharmacol. 2009;63(2):261–6.
Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250(4):558–66.
Al-Hilli Z, Hieken TJ, Hoskin TL, Heins CN, Boughey JC. Impact of neoadjuvant chemotherapy on pathologic axillary nodal status in HER-2 positive patients presenting with clinically node-negative disease. J Surg Oncol. 2015;112(5):453–7.
Acknowledgement
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study were derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Hilli, Z., Hoskin, T.L., Day, C.N. et al. Impact of Neoadjuvant Chemotherapy on Nodal Disease and Nodal Surgery by Tumor Subtype. Ann Surg Oncol 25, 482–493 (2018). https://doi.org/10.1245/s10434-017-6263-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6263-y