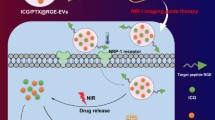
Abstract
Glioma, in which a malignant tumor cell occurs in neural mesenchymal cells, has a rapid progression and poor prognosis, which is still far from desirable in clinical treatments. We developed a lab-on-a-chip (LOC) device for the rapid and efficient preparation of vitexin/indocyanine green (ICG) liposomes. Vitexin could be released from liposome to kill cancer cell, which can potentially improve the glioma therapeutic effect and reduce the treatment time through synergistic photodynamic/photothermal therapies (PDT/PTT). The vitexin/ICG liposome was fabricated via LOC and its physicochemical property and release in vitro were evaluated. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method and live/dead staining were used to examine the enhanced antitumor effect of vitexin/ICG liposome in cooperation with PDT/PTT, while the related mechanism was explored by flow cytometry and western blot. The results were as follows: (1) The prepared vitexin/ICG liposome was smaller in size, homogenous in particle size distribution with significant low polydispersity index (PDI), and enhanced cumulative release in vitro. (2) We found that the formulated liposome presented strong cancer cell inhibition and suppression of its migration in a dose-dependent manner. (3) Further mechanistic studies showed that liposome combined with near-infrared irradiation could significantly upregulate levels of B cell lymphoma 2-associated X (Bax) protein and decrease B cell lymphoma 2 (Bcl-2) at protein levels. The vitexin/ICG liposomes prepared based on a simple LOC platform can effectively enhance the solubility of insoluble drugs, and the combined effect of PTT/PDT can effectively increase their antitumor effect, which provides a simple and valid method for the clinical translation of liposomes.
Graphical Abstract











Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Li H, Liu Y, Jin H, Liu S, Fang S, Wang C, et al. Separation of vitexin-4″-O-glucoside and vitexin-2″-O-rhamnoside from hawthorn leaves extracts using macroporous resins. J Chromatogr B Anal Technol Biomed Life Sci. 2015;1007:23–9. https://doi.org/10.1016/j.jchromb.2015.10.043.
Vlaisavljevic S, Sibul F, Sinka I, Zupko I, Ocsovszki I, Jovanovic-Santa S. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Ind Crops Prod. 2018;112:217–24. https://doi.org/10.1016/j.indcrop.2017.11.050.
Yu X, Huang J, Xu D, Xie Z, Xie Z, Xu X. Isolation and purification of orientin and vitexin from Trollius chinensis Bunge by high-speed counter-current chromatography. Nat Prod Res. 2014;28(9):674–6. https://doi.org/10.1080/14786419.2014.891111.
Yuan M, Wang R, Liu L, Yang X, Peng Y, Sun Z. Contribution evaluation of the floral parts to orientin and vitexin concentrations in the flowers of Trollius chinensis. Chin J Nat Med. 2013;11(6):699–704. https://doi.org/10.1016/s1875-5364(13)60082-5.
Fu Y, Zu Y, Liu W, Hou C, Chen L, Li S, et al. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J Chromatogr A. 2007;1139(2):206–13. https://doi.org/10.1016/j.chroma.2006.11.015.
Kim J, Lee B, Kim J, Sim G, Lee D, Lee K, et al. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28(2):195–202. https://doi.org/10.1007/bf02977715.
Kassuya RM, Dos Santos E, Bosso FH, Pedroso TF, Marinho JVN, Salvador MJ, et al. Anti-inflammatory properties of ethanolic extract and 2’’-O-beta-D-glucopyranosyl-vitexin obtained from Alternanthera tenella colla whole plant. Inflammation. 2021;44(4):1540–52. https://doi.org/10.1007/s10753-021-01438-7.
Li XS, Tang XY, Su W, Li X. Vitexin protects melanocytes from oxidative stress via activating MAPK-Nrf2/ARE pathway. Immunopharmacol Immunotoxicol. 2020;42(6):594–603. https://doi.org/10.1080/08923973.2020.1835952.
Prabhakar M, Bano H, Kumar I, Shamsi M, Khan M. Pharmacological investigations on vitexin. Planta Med. 1981;43(4):396–403. https://doi.org/10.1055/s-2007-971532.
de Oliveira D, da Silva C, Iglesias B, Beleboni R. Vitexin possesses anticonvulsant and anxiolytic-like effects in murine animal models. Front Pharmacol. 2020;11:1181. https://doi.org/10.3389/fphar.2020.01181.
Liu X, Jiang Q, Liu H, Luo S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol Res. 2019;52(1):7. https://doi.org/10.1186/s40659-019-0214-y.
Papi A, Farabegoli F, Iori R, Orlandi M, De Nicola G, Bagatta M, et al. Vitexin-2-O-xyloside, raphasatin and (-)-epigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013;138:1521–30. https://doi.org/10.1016/j.foodchem.2012.11.112.
Lee J, Mohan C, Shanmugam M, Rangappa S, Sethi G, Siveen K, et al. Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie. 2020;175:58–68. https://doi.org/10.1016/j.biochi.2020.05.006.
Yang S, Liao P, Pan Y, Chen S, Chou S, Chou M. The novel p53-dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells. Phytother Res PTR. 2013;27(8):1154–61. https://doi.org/10.1002/ptr.4841.
Tan Z, Zhang Y, Deng J, Zeng G, Zhang Y. Purified vitexin compound 1 suppresses tumor growth and induces cell apoptosis in a mouse model of human choriocarcinoma. International Journal of Gynecological Cancer : Official Journal of the International Gynecological Cancer Society. Int J Gynecol Cancer. 2012;22(3):360-6. https://doi.org/10.1097/IGC.0b013e31823de844.
Li Y, Sun Q, Li H, Yang B, Wang M. Vitexin suppresses renal cell carcinoma by regulating mTOR pathways. Transl Androl Urol. 2020;9(4):1700–11. https://doi.org/10.21037/tau-20-1094.
Wang W, Cheng H, Gu X, Yin X. The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF-κB signaling in nasopharyngeal carcinoma. Onco Targets Ther. 2019;12:4461–8. https://doi.org/10.2147/ott.S210077.
Min JW, Hu JJ, He M, Sanchez RM, Huang WX, Liu YQ, et al. Vitexin reduces hypoxia-ischemia neonatal brain injury by the inhibition of HIF-1 alpha in a rat pup model. Neuropharmacology. 2015;99:38–50. https://doi.org/10.1016/j.neuropharm.2015.07.007.
Abdel Razek A, Talaat M, El-Serougy L, Gaballa G, Abdelsalam M. Clinical applications of arterial spin labeling in brain tumors. J Comput Assist Tomogr. 2019;43(4):525–32. https://doi.org/10.1097/rct.0000000000000873.
Zu YG, Zhang Q, Zhao XH, Wang D, Li W, Sui XY, et al. Preparation and characterization of vitexin powder micronized by a supercritical antisolvent (SAS) process. Powder Technol. 2012;228:47–55. https://doi.org/10.1016/j.powtec.2012.04.048.
Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23(4):907.
Kraft JC, Freeling JP, Wang ZY, Ho RJY. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29–52. https://doi.org/10.1002/jps.23773.
Wang GW, Wu BH, Li QY, Chen SQ, Jin XQ, Liu YJ, et al. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small. 2020;16(44):14. https://doi.org/10.1002/smll.202004172.
Rossier O, Cuvelier D, Borghi N, Puech PH, Derenyi I, Buguin A, et al. Giant vesicles under flows: extrusion and retraction of tubes. Langmuir. 2003;19(3):575–84. https://doi.org/10.1021/la026236t.
Pott T, Bouvrais H, Meleard P. Giant unilamellar vesicle formation under physiologically relevant conditions. Chem Phys Lipids. 2008;154(2):115–9. https://doi.org/10.1016/j.chemphyslip.2008.03.008.
Long MS, Cans A-S, Keating CD. Budding and asymmetric protein microcompartmentation in giant vesicles containing two aqueous phases. J Am Chem Soc. 2008;130(2):756–62. https://doi.org/10.1021/ja077439c.
Wischke C. Concepts for efficient preparation of particulate polymer carrier systems by droplet -based microfluidics. Int J Pharm. 2020;584:19. https://doi.org/10.1016/j.ijpharm.2020.119401.
Koh CG, Zhang XL, Liu SJ, Golan S, Yu B, Yang XJ, et al. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J Control Release. 2010;141(1):62–9. https://doi.org/10.1016/j.jconrel.2009.08.019.
Essa D, Choonara YE, Kondiah PPD, Pillay V. Comparative nanofabrication of PLGA-chitosan-PEG systems employing microfluidics and emulsification solvent evaporation techniques. Polymers. 2020;12(9). https://doi.org/10.3390/polym12091882.
Jahn A, Vreeland W, DeVoe D, Locascio L, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23(11):6289–93. https://doi.org/10.1021/la070051a.
Hong JS, Stavis SM, Lacerda SHD, Locascio LE, Raghavan SR, Gaitan M. Microfluidic directed self-assembly of liposome-hydrogel hybrid nanoparticles. Langmuir. 2010;26(13):11581–8. https://doi.org/10.1021/la100879p.
Liu ZM, Du Y, Pang Y. Generation of water-in-oil-in-water (W/O/W) double emulsions by microfluidics. Chin J Anal Chem. 2018;46(3):324–31. https://doi.org/10.11895/j.issn.0253-3820.171187.
Shah VM, Nguyen DX, Patel P, Cote B, Al-Fatease A, Pham Y, et al. Liposomes produced by microfluidics and extrusion: a comparison for scale-up purposes. Nanomed-Nanotechnol Biol Med. 2019;18:146–56. https://doi.org/10.1016/j.nano.2019.02.019.
Jang B, Park JY, Tung CH, Kim IH, Choi Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano. 2011;5(2):1086–94. https://doi.org/10.1021/nn102722z.
Chen Q, Wang C, Zhan ZX, He WW, Cheng ZP, Li YY, et al. Near-infrared dye bound albumin with separated imaging and therapy wavelength channels for imaging-guided photothermal therapy. Biomaterials. 2014;35(28):8206–14. https://doi.org/10.1016/j.biomaterials.2014.06.013.
Chen R, Wang X, Yao XK, Zheng XC, Wang J, Jiang XQ. Near-IR-triggered photothermal/photodynamic dual-modality therapy system via chitosan hybrid nanospheres. Biomaterials. 2013;34(33):8314–22. https://doi.org/10.1016/j.biomaterials.2013.07.034.
Seo SH, Kim BM, Joe A, Han HW, Chen XY, Cheng Z, et al. NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod@SiO2 nanocomposites. Biomaterials. 2014;35(10):3309–18. https://doi.org/10.1016/j.biomaterials.2013.12.066.
Taylor JS, Zeki J, Ikegaki N, Chen LHL, Chiu B. Combined application of Indocyanine green (ICG) and laser lead to targeted tumor cell destruction. J Pediatr Surg. 2018;53(12):2475–9. https://doi.org/10.1016/j.jpedsurg.2018.08.013.
Carr JA, Franke D, Caram JR, Perkinson CF, Saif M, Askoxylakis V, et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc Natl Acad Sci USA. 2018;115(17):4465–70. https://doi.org/10.1073/pnas.1718917115.
Xue P, Yang RH, Sun LH, Li Q, Zhang L, Xu ZG, et al. Indocyanine green-conjugated magnetic Prussian blue nanoparticles for synchronous photothermal/photodynamic tumor therapy. Nano-Micro Lett. 2018;10(4). https://doi.org/10.1007/s40820-018-0227-z.
Huang XQ, Wu JR, He MY, Hou XY, Wang Y, Cai XR, et al. Combined cancer chemo-photodynamic and photothermal therapy based on ICG/PDA/TPZ-loaded nanoparticles. Mol Pharm. 2019;16(5):2172–83. https://doi.org/10.1021/acs.molpharmaceut.9b00119.
Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101(3):529–33. https://doi.org/10.1016/S0161-6420(94)31303-0.
Jiang XY, Du BJ, Huang YY, Yu MX, Zheng J. Cancer photothermal therapy with ICG-conjugated gold nanoclusters. Bioconjug Chem. 2020;31(5):1522–8. https://doi.org/10.1021/acs.bioconjchem.0c00172.
van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158(1):148–55. https://doi.org/10.1016/j.jconrel.2011.10.021.
Velpula A, Jukanti R, Janga KY, Sunkavalli S, Bandari S, Kandadi P, et al. Proliposome powders for enhanced intestinal absorption and bioavailability of raloxifene hydrochloride: effect of surface charge. Drug Dev Ind Pharm. 2013;39(12):1895–906. https://doi.org/10.3109/03639045.2012.670641.
Liu J, Wang QL, Omari-Siaw E, Adu-Frimpong M, Liu J, Xu XM, et al. Enhanced oral bioavailability of Bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: formulation design, in vitro and in vivo evaluation. Int J Pharm. 2020;590. https://doi.org/10.1016/j.ijpharm.2020.119887.
Zhang XW, Lue SW, Han JH, Sun S, Wang LM, Li YJ. Preparation, characterization and in vivo distribution of solid lipid nanoparticles loaded with syringopicroside. Pharmazie. 2011;66(6):404–7. https://doi.org/10.1691/ph.2011.0350.
Bresseleers J, Bagheri M, Lebleu C, Lecommandoux S, Sandre O, Pijpers IAB, et al. Tuning size and morphology of mPEG-b-p(HPMA-Bz) copolymer self-assemblies using microfluidics. Polymers. 2020;12(11). https://doi.org/10.3390/polym12112572.
Wang QL, Wei QY, Yang QX, Cao X, Li Q, Shi F, et al. A novel formulation of 6 -gingerol: proliposomes with enhanced oral bioavailability and antitumor effect. Int J Pharm. 2018;535(1–2):308–15. https://doi.org/10.1016/j.ijpharm.2017.11.006.
Ge ZM, Wang QL, Zhu Q, Yusif M, Yu JN, Xu XM. Improved oral bioavailability, cellular uptake, and cytotoxic activity of zingerone via nano-micelles drug delivery system. J Microencapsul. 2021;38(6):394–404. https://doi.org/10.1080/02652048.2021.1957036.
Hood R, Shao C, Omiatek D, Vreeland W, DeVoe D. Microfluidic synthesis of PEG- and folate-conjugated liposomes for one-step formation of targeted stealth nanocarriers. Pharm Res. 2013;30(6):1597–607. https://doi.org/10.1007/s11095-013-0998-3.
Martín-Banderas L, González-Prieto R, Rodríguez-Gil A, Fernández-Arévalo M, Flores-Mosquera M, Chávez S, et al. Application of flow focusing to the break-up of a magnetite suspension jet for the production of paramagnetic microparticles. Chemical Engineering Science. #N/A. 2011;2011:527437. https://doi.org/10.1155/2011/527437.
Yu B, Lee RJ, Lee LJ. Microfluidic methods for production of liposomes. Methods Enzymol. 2009;465:129–41. https://doi.org/10.1016/s0076-6879(09)65007-2.
Chen R, Wulff JE, Moffitt MG. Microfluidic processing approach to controlling drug delivery properties of curcumin-loaded block copolymer nanoparticles. Mol Pharm. 2018;15(10):4517–28. https://doi.org/10.1021/acs.molpharmaceut.8b00529.
Zhu Y, Wang MM, Zhang JJ, Peng W, Firempong CK, Deng WW, et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: preparation, in vitro drug release and pharmacokinetics in rats. Arch Pharm Res. 2015;38(4):512–21. https://doi.org/10.1007/s12272-014-0481-7.
Li R, Zhang LY, Li ZJ, Xue CH, Dong P, Huang QR, et al. Characterization and absorption kinetics of a novel multifunctional nanoliposome stabilized by sea cucumber saponins instead of cholesterol. J Agric Food Chem. 2020;68(2):642–51. https://doi.org/10.1021/acs.jafc.9b06460.
Wiącek AE, Jurak M, Ładniak A, Przykaza K, Szafran K. Cyclosporine CsA—the physicochemical characterization of liposomal and colloidal systems. Colloids Interfaces. 2020;4(4):46.
Zhang WD, Peng FQ, Zhou TT, Huang YF, Zhang L, Ye P, et al. Targeted delivery of chemically modified anti-miR-221 to hepatocellular carcinoma with negatively charged liposomes. Int J Nanomed. 2015;10:4825–36. https://doi.org/10.2147/ijn.S79598.
Wang Q, Wei C, Weng W, Bao R, Adu-Frimpong M, Toreniyazov E, et al. Enhancement of oral bioavailability and hypoglycemic activity of liquiritin-loaded precursor liposome. Int J Pharm. 2021;592:120036. https://doi.org/10.1016/j.ijpharm.2020.120036.
Wang YW, Wang SC, Firempong CK, Zhang HY, Wang MM, Zhang Y, et al. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: preparation and in vitro and in vivo evaluations. AAPS PharmSciTech. 2017;18(3):586–94. https://doi.org/10.1208/s12249-016-0537-8.
Moslehi M, Mortazavi SAR, Azadi A, Fateh S, Hamidi M, Foroutan SM. Preparation, optimization and characterization of chitosan-coated liposomes for solubility enhancement of furosemide: a model BCS IV drug. Iran J Pharm Res IJPR. 2020;19(1):366–82. https://doi.org/10.22037/ijpr.2019.111834.13384.
Zhang B, Pan WL, Deng YX, He HB, Gou JX, Wang YJ, et al. Panax quinquefoliumsaponin liposomes prepared by passive drug loading for improving intestinal absorption. Drug Dev Ind Pharm. 2020;46(10):1684–94. https://doi.org/10.1080/03639045.2020.1820036.
Liu Y, Sun C, Li W, Adu-Frimpong M, Wang Q, Yu J, et al. Preparation and characterization of syringic acid-loaded TPGS liposome with enhanced oral bioavailability and in vivo antioxidant efficiency. AAPS PharmSciTech. 2019;20(3):98. https://doi.org/10.1208/s12249-019-1290-6.
Michelon M, Oliveira DRB, de Figueiredo Furtado G, Gaziola de la Torre L, Cunha RL. High-throughput continuous production of liposomes using hydrodynamic flow-focusing microfluidic devices. Colloids Surf B Biointerfaces. 2017;156:349–57. https://doi.org/10.1016/j.colsurfb.2017.05.033.
Bottaro E, Nastruzzi C. “Off-the-shelf” microfluidic devices for the production of liposomes for drug delivery. Mater Sci Eng C Mater Biol Appl. 2016;64:29–33. https://doi.org/10.1016/j.msec.2016.03.056.
Zhang K, Wang Q, Yang Q, Wei Q, Man N, Adu-Frimpong M, et al. Enhancement of oral bioavailability and anti-hyperuricemic activity of isoliquiritigenin via self-microemulsifying drug delivery system. AAPS PharmSciTech. 2019;20(5):218. https://doi.org/10.1208/s12249-019-1421-0.
Saxena V, Sadoqi M, Shao J. Degradation kinetics of indocyanine green in aqueous solution. J Pharm Sci. 2003;92(10):2090–7. https://doi.org/10.1002/jps.10470.
Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–97. https://doi.org/10.1016/S1470-2045(02)00818-5.
Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56. https://doi.org/10.1016/S1040-8428(01)00179-2.
Luo L, Zeng F, Xie J, Fan J, Xiao S, Wang Z, et al. A RBC membrane-camouflaged biomimetic nanoplatform for enhanced chemo-photothermal therapy of cervical cancer. J Mater Chem B. 2020;8(18):4080–92. https://doi.org/10.1039/c9tb02937k.
Shao W, Yang C, Li F, Wu J, Wang N, Ding Q, et al. Molecular design of conjugated small molecule nanoparticles for synergistically enhanced PTT/PDT. Nano-Micro Lett. 2020;12(11).
Li JQ, Qi XT, Ye PK, Yang M, Xie M. Construction of WS2/Au-lipid drug delivery system for multiple combined therapy of tumor. J Drug Deliv Sci Technol. 2022;76. https://doi.org/10.1016/j.jddst.2022.103747.
Zheng XH, Zhou FF, Wu BY, Chen WR, Xing D. Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection. Mol Pharm. 2012;9(3):514–22. https://doi.org/10.1021/mp200526m.
Nomikou N, Sterrett C, Arthur C, McCaughan B, Callan JF, McHale AP. The effects of ultrasound and light on indocyanine-green-treated tumour cells and tissues. ChemMedChem. 2012;7(8):1465–71. https://doi.org/10.1002/cmdc.201200233.
Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, et al. Single-step assembly of DOX/ICG loaded lipid–polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013;7(3):2056–67. https://doi.org/10.1021/nn400334y.
Zhou J, Meng LC, Sun C, Ye WR, Chen CQ, Du B. A “protective umbrella” nanoplatform for loading ICG and multi-modal imaging-guided phototherapy. Nanomed-Nanotechnol Biol Med. 2018;14(2):289–301. https://doi.org/10.1016/j.nano.2017.09.009.
Garner TP, Reyna DE, Priyadarshi A, Chen HC, Li S, Wu Y, et al. An autoinhibited dimeric form of BAX regulates the BAX activation pathway. Mol Cell. 2016;63(3):485–97. https://doi.org/10.1016/j.molcel.2016.06.010.
Jensen K, WuWong DJ, Wong S, Matsuyama M, Matsuyama S. Pharmacological inhibition of Bax-induced cell death: Bax-inhibiting peptides and small compounds inhibiting Bax. Exp Biol Med (Maywood). 2019;244(8):621–9. https://doi.org/10.1177/1535370219833624.
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. https://doi.org/10.1016/j.molcel.2010.01.025.
Rudner J, Jendrossek V, Belka C. New insights in the role of Bcl-2 Bcl-2 and the endoplasmic reticulum. Apoptosis. 2002;7(5):441–7. https://doi.org/10.1023/a:1020087108926.
Shi WW, Cao X, Liu Q, Zhu Q, Liu K, Deng TW, et al. Hybrid membrane-derived nanoparticles for isoliquiritin enhanced glioma therapy. Pharmaceuticals. 2022;15(9). https://doi.org/10.3390/ph15091059.
Funding
This work was funded by the National Key R&D Program of China (2018YFE0208600), Key Planning Social Development Projects of Zhenjiang in Jiangsu Province (SH2021024), National Natural Science Foundation of China (81720108030, 8217131836 and 82173785), Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (18KJB360001), Natural Science Foundation of Jiangsu Province (BK20180866), and Postdoctoral Research Fund of Jiangsu Province in 2021 category A (2021K010A). Also, we thank the Ethics Committee of University for guiding us on animal experiment and to the Institute of Pharmacy, Jiangsu University, Zhenjiang, Jiangsu, China, for the necessary facility support to generate the manuscript.
Author information
Authors and Affiliations
Contributions
Cao Xia, Ximing Xu, and Qilong Wang contributed to the conceptualization; Qi Liu, Wenwan Shi, Hui Yuan, and Xuedi Weng contributed to the data curation and Methodology. Qi Liu, Kai liu, Tianwen Deng, and Yihong Gao contributed to the formal analysis and validation. Qingtong Yu, WENWEN Deng, and Gao Xiao contributed to the supervision. Xia Cao, Qi Liu, Michael Adu-Frimpong, Qilong Wang, and Gao Xiao contributed to the writing. Xia Cao, Jiangnan Yu, and Ximing Xu contributed to the funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, X., Liu, Q., Adu-Frimpong, M. et al. Microfluidic Generation of Near-Infrared Photothermal Vitexin/ICG Liposome with Amplified Photodynamic Therapy. AAPS PharmSciTech 24, 82 (2023). https://doi.org/10.1208/s12249-023-02539-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02539-2