Abstract
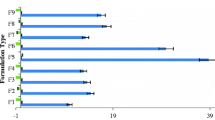
Herein, we developed an ethosomal hydrogel based on three types of ethosomes: simple, mixed (surfactant-based micelles and lipid vesicles) or binary (comprising two type of alcohols). Ethanol injection was employed for vesicles preparation, and sodium alginate, as gelling agent. We purposed the local-transdermal administration of the off-the-shelf retinoid fenretinide (FENR) for chemoprevention of breast cancer. Rheograms and flow index values for alginate dispersion (without ethosomes) and hydrogels containing simple, mixed or binary ethosomes suggested pseudoplastic behavior. An increase in the apparent viscosity was observed upon ethosome incorporation. The ethosomal hydrogel displayed increased bioadhesion compared to the alginate dispersion, suggesting that the lipid vesicles contribute to the gelling and bioadhesion processes. In the Hen’s Egg Test–Chorioallantoic Membrane model, few spots of lysis and hemorrhage were observed for formulations containing simple (score of 2) and mixed vesicles (score 4), but not for the hydrogel based on the binary system, indicating its lower irritation potential. The binary ethosomal hydrogel provided a slower FENR in vitro release and delivered 2.6-fold less drug into viable skin layers compared to the ethosome dispersion, supporting the ability of the gel matrix to slow down drug release. The ethosomal hydrogel decreased by ~ five-fold the IC50 values of FENR in MCF-7 cells. In conclusion, binary ethosomal gels presented technological advantages, provided sustained drug release and skin penetration, and did not preclude drug cytotoxic effects, supporting their potential applicability as topical chemopreventive systems.







Similar content being viewed by others
References
Veronesi U, Mariani L, Decensi A, Formelli F, Camerini T, Miceli R, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–71.
Cooper JP, Reynolds CP, Cho H, Kang MH. Clinical development of fenretinide as an antineoplastic drug: pharmacology perspectives. Exp Biol Med. 2017;242:1178–84.
Apolinário AC, Hauschke L, Nunes JR, Lopes LB. Towards nanoformulations for skin delivery of poorly soluble API: what does indeed matter? J Drug Deliv Sci Technol. 2020;60:1–13.
Mojeiko G, Passos JS, Apolinario AC, Lopes LB. Topical transdermal chemoprevention of breast cancer: where will nanomedical approaches deliver us? Nanomedicine. 2021;16:1713–31.
Narvekar M, Xue HY, Eoh JY, Wong HL. Nanocarrier for poorly water-soluble anticancer drugs—barriers of translation and solutions. AAPS PharmSciTech. 2014;15:822–33.
Lee O, Khan SA. Novel routes for administering chemoprevention: local transdermal therapy to the breasts. Semin Oncol. 2016;43:107–15. https://doi.org/10.1053/j.seminoncol.2015.09.003.
Lee O, Ivancic D, Allu S, Shidfar A, Kenney K, Helenowski I, et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol. 2015;76:1235–46.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review development. Pharmaceutics. 2017;9:1–33.
Touti R, Noun M, Guimberteau F, Lecomte S, Faure C. What is the fate of multi-lamellar liposomes of controlled size, charge and elasticity in artificial and animal skin? Eur J Pharm Biopharm. 2020;151:18–31. https://doi.org/10.1016/j.ejpb.2020.03.017.
Apolinário AC, Hauschke L, Nunes JR, Lopes LB. Lipid nanovesicles for biomedical applications: ‘What is in a name’? Prog Lipid Res. 2021;82:101096.
Apolinário AC, Hauschke L, Nunes JR, Lourenço Felipe Rebello, Lopes LB. Design of multifunctional ethosomes for topical fenretinide delivery and breast cancer chemoprevention. Colloids Surfaces A Physicochem Eng Asp. 2021;623:126745.
Rehman K, Zulfakar MH. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm. 2014;40:433–40.
ICCVAM. Test method evaluation report: current validation status of in vitro test methods proposed for identifying eye injury hazard potential of chemicals and products (volume 2) Interagency Coordinating Committee on the Validation of Alternative Methods Nationa. 2010;2. Available from: https://ntp.niehs.nih.gov/iccvam/docs/ocutox_docs/invitro-2010/tmer-vol2.pdf
Hosmer JM, Steiner AA, Lopes LB. Lamellar liquid crystalline phases for cutaneous delivery of paclitaxel: impact of the monoglyceride. Pharm Res. 2013;30:694–706.
Trivedi R, Umekar M, Kotagale N, Bonde S, Taksande J. Design, evaluation and in vivo pharmacokinetic study of a cationic flexible liposomes for enhanced transdermal delivery of pramipexole. J Drug Deliv Sci Technol. 2021;61: 102313. https://doi.org/10.1016/j.jddst.2020.102313.
Shah H, Nair AB, Shah J, Jacob S, Bharadia P, Haroun M. Proniosomal vesicles as an effective strategy to optimize naproxen transdermal delivery. J Drug Deliv Sci Technol. 2021;63: 102479. https://doi.org/10.1016/j.jddst.2021.102479.
Marwah MK, Shokr H, Sanchez-Aranguren L, Badhan RKS, Wang K, Ahmad S. Transdermal delivery of a hydrogen sulphide donor, ADT-OH using aqueous gel formulations for the treatment of impaired vascular function: an ex vivo study. Pharm Res Pharmaceutical Research. 2022;39:341–52.
Kuznetsova DA, Vasileva LA, Gaynanova GA, Vasilieva EA, Lenina OA, Nizameev IR, et al. Cationic liposomes mediated transdermal delivery of meloxicam and ketoprofen: Optimization of the composition, in vitro and in vivo assessment of efficiency. Int J Pharm. 2021;605:120803. https://doi.org/10.1016/j.ijpharm.2021.120803.
Altamimi MA, Hussain A, Alrajhi M, Alshehri S, Imam SS, Qamar W. Luteolin-loaded elastic liposomes for transdermal delivery to control breast cancer: in vitro and ex vivo evaluations. Pharmaceuticals. 2021;14.
Altamimi MA, Hussain A, Alshehri S, Imam SS. Experimental design based optimization and ex vivo permeation of desmopressin acetate loaded elastic liposomes using rat skin. Pharmaceutics. 2021;13.
Ali AA, Hassan AH, Eissa EM, Aboud HM. Response surface optimization of ultra-elastic nanovesicles loaded with deflazacort tailored for transdermal delivery: accentuated bioavailability and anti-inflammatory efficacy. Int J Nanomedicine. 2021;16:591–607.
Jain A, Jain SK. In vitro release kinetics model fitting of liposomes: an insight. Chem Phys Lipids. 2016;201:28–40. https://doi.org/10.1016/j.chemphyslip.2016.10.005.
Haidar ZS, Hamdy RC, Tabrizian M. Protein release kinetics for core-shell hybrid nanoparticles based on the layer-by-layer assembly of alginate and chitosan on liposomes. Biomaterials. 2008;29:1207–15.
Fonseca-Santos B, Satake CY, Calixto GMF, Dos Santos AM, Chorilli M. Trans-resveratrol-loaded nonionic lamellar liquid-crystalline systems: structural, rheological, mechanical, textural, and bioadhesive characterization and evaluation of in vivo anti-inflammatory activity. Int J Nanomedicine. 2017;12:6883–93.
Carvalho V, Lemos DP, Vieira CS, Migotto A, Lopes LB. Potential of non-aqueous microemulsions to improve the delivery of lipophilic drugs to the skin. AAPS PharmSciTech AAPS PharmSciTech. 2017;18:1739–49.
Pepe D, Carvalho VFM, McCall M, De Lemos DP, Lopes LB. Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel. Int J Nanomedicine. 2016;11:2009–19.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Salata GC, Malagó ID, Carvalho Dartora VFM, Marçal Pessoa AF, Fantini MC de A, Costa SKP, et al. Microemulsion for prolonged release of fenretinide in the mammary tissue and prevention of breast cancer development. Mol Pharm. American Chemical Society (ACS); 2021;
Shapiro YE. Structure and dynamics of hydrogels and organogels: an NMR spectroscopy approach. Prog Polym Sci. 2011;36:1184–253. https://doi.org/10.1016/j.progpolymsci.2011.04.002.
Ahmed EM. Hydrogel : Preparation, characterization, and applications: a review. J Adv Res Cairo University. 2015;6:105–21. https://doi.org/10.1016/j.jare.2013.07.006.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
El Kechai N, Bochot A, Huang N, Nguyen Y, Ferrary E, Agnely F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int J Pharm. 2015;487:187–96. https://doi.org/10.1016/j.ijpharm.2015.04.019.
Heatley F, Scott JE. A water molecule participates in the secondary structure of hyaluronan. Biochem J. 1988;254:489–93.
Ionov R, El-Abed A, Goldmann M, Peretti P. Interactions of lipid monolayers with the natural biopolymer hyaluronic acid. Biochim Biophys Acta - Biomembr. 2004;1667:200–7.
Duro-Castano A, Poma A, Pessoa A, Bueno CZ, Apolina AC, Rangel-yagui CO, et al. L - asparaginase encapsulation into asymmetric permeable polymersomes. Acs Macro Lett. 2020;9:1471–7.
Devi N, Kakati DK. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J Food Eng Elsevier Ltd. 2013;117:193–204. https://doi.org/10.1016/j.jfoodeng.2013.02.018.
Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014;460:280–8. https://doi.org/10.1016/j.ijpharm.2013.11.017.
Junqueira Garcia MT, Pedralino Gonçalves T, São Félix Martins É, Silva Martins T, de Abreu Carvalho, Fantini M, RegaziMinarini PR, et al. Improvement of cutaneous delivery of methylene blue by liquid crystals. Int J Pharm. 2018;548:454–65.
Mojeiko G, de Brito M, Salata GC, Lopes LB. Combination of microneedles and microemulsions to increase celecoxib topical delivery for potential application in chemoprevention of breast cancer. Int J Pharm Elsevier. 2019;560:365–76. https://doi.org/10.1016/j.ijpharm.2019.02.011.
U.S. Food and Drug Administration. Inactive ingredient search for approved drug products [Internet]. U.S. Food Drug Adm. 2020 [cited 2021 Mar 15]. Available from: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm
Camelo SRP, Franceschi S, Perez E, Fullana SG, Ré MI. Factors influencing the erosion rate and the drug release kinetics from organogels designed as matrices for oral controlled release of a hydrophobic drug. Drug Dev Ind Pharm. 2016;42:985–97.
Mircioiu C, Voicu V, Anuta V, Tudose A, Celia C, Paolino D, et al. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019;11:1–45.
Marcos LB. Mathematical models of drug release. In: Bruschi ML, editor. Strategies to modify drug release from pharmaceutical systems. Elsevier; 2015. p. 63–86.
Shetty S, Jose J, Kumar L, Charyulu RN. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J Cosmet Dermatol. 2019;18:862–9.
Glavas-Dodov M, Fredro-Kumbaradzi E, Goracinova K, Simonoska M, Calis S, Trajkovic-Jolevska S, et al. The effects of lyophilization on the stability of liposomes containing 5-FU. Int J Pharm. 2005;291:79–86.
Fathalla D, Youssef EMK, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12:1–24.
Yoon HY, Kwak SS, Jang MH, Kang MH, Sung SW, Kim CH, et al. Docetaxel-loaded RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes: drug release, cytotoxicity, and antitumor efficacy. Int J Pharm. 2017;523:229–37. https://doi.org/10.1016/j.ijpharm.2017.03.045.
Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57:1556–68.
Woodley J. Bioadhesion new possibilities for drug administration? Clin Pharmacokinet. 2001;40:77–84.
Jin SG, Yousaf AM, Kim KS, Kim DW, Kim DS, Kim JK, et al. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int J Pharm. 2016;501:160–6. https://doi.org/10.1016/j.ijpharm.2016.01.044.
Frank LA, Chaves PS, D’Amore CM, Contri RV, Frank AG, Beck RCR, et al. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: increasing penetration and adhesion of imiquimod in vaginal tissue. Eur J Pharm Biopharm. 2017;114:202–12. https://doi.org/10.1016/j.ejpb.2017.01.021.
Izquierdo MC, Lillo CR, Bucci P, Gómez GE, Martínez L, Alonso V, et al. Comparative skin penetration profiles of formulations including ultradeformable liposomes as potential nanocosmeceutical carriers. J Cosmet Dermatol. 2020;19:3127–37.
Niu X, Zhang D, Bian Q, Feng X, Li H, Rao Y. Mechanism investigation of ethosomes transdermal permeation. Int J Pharm X. 2019;1:100027. https://doi.org/10.1016/j.ijpx.2019.100027.
Zhang Y, Ng W, Hu J, Saleh S, Ge Y, Xu H. Formulation and in vitro stability evaluation of ethosomal carbomer hydrogel for transdermal vaccine delivery. Colloids Surfaces B Biointerfaces. 2018;163:184–91. https://doi.org/10.1016/j.colsurfb.2017.12.031.
Mishra A, Pandey VK, Shankar BS, Melo JS. Spray drying as an efficient route for synthesis of silica nanoparticles-sodium alginate biohybrid drug carrier of doxorubicin. Colloids Surfaces B Biointerfaces. 2021;197: 111445. https://doi.org/10.1016/j.colsurfb.2020.111445.
Gong T. Improvement of in vitro anticancer activity of doxorubicin by sodium alginate nanoparticles delivery. J Pharm Biomed Sci. 2018;08:89–93.
Rossi T, Iannuccelli V, Coppi G, Bruni E, Baggio G. Role of the pharmaceutical excipients in the tamoxifen activity on MCF-7 and vero cell cultures. Anticancer Res. 2009;29:4529–33.
Acknowledgements
The authors would like to acknowledge Dr. Leticia Costa-Lotufo (Institute of Biomedical Sciences, University of Sao Paulo) for use of the cell culture facility.
Funding
This study was supported by São Paulo Research Foundation (FAPESP, grant# 2018/ 13877–1) and CAPES (finance code 001). Fellowships from FAPESP (grant# 2018/14375–0 to A.C. Apolinário) and National Council of Technological and Scientific Development (306866/2020–0 to L.B. Lopes) are greatly appreciated. This study is part of the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach INCT-NANOFARMA, which is supported by FAPESP (grant #2014/50928–2) and CNPq (grant # 465687/2014–8). The authors are grateful to CENTD for support with the confocal microscopy facility. CENTD is supported by São Paulo Research Foundation (FAPESP) grant number 2020/13139–0 (FAPESP/GSK/Instituto Butantan).
Author information
Authors and Affiliations
Contributions
Conceptualization—A.C.A. and L.B.L.; confocal microscopy assay—M.M.S.; HET-CAM and rheology assay—G.C.S.; bioadhesion: M.C.; writing—original draft preparation—A.C.A., M.C. and L.B.L.; funding acquisition—L.B.L.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors filed for a patent application in Brazil in a topic related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Apolinário, A.C., Salata, G.C., de Souza, M.M. et al. Rethinking Breast Cancer Chemoprevention: Technological Advantages and Enhanced Performance of a Nanoethosomal-Based Hydrogel for Topical Administration of Fenretinide. AAPS PharmSciTech 23, 104 (2022). https://doi.org/10.1208/s12249-022-02257-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02257-1