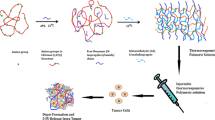
Abstract
In the present study, different in situ hydrogel formulations of docetaxel (DTX) based on biocompatible polymers such as Hyaluronic Acid (HA), poloxamer-407, chitosan and gellan gum were formulated to increase its therapeutic efficacy and reduce toxicity. DTX was loaded in nanovesicles (20 mg/mL equivalent to commercial strength) and further incorporated into the hydrogel bases to possess a dual rationale of protection against burst release and enhanced solubility of the drug. The optimized hydrogel formulation (NV-TPGS-3-GG-4) showed ideal rheological behavior and in situ characteristics at 37±0.5°C with sustained release of more than 144 h. The optimized formulation had instant in vitro gelation (2.8±0.3 min) with good injectability in comparison to the conventional commercial DTX injectable formulation having instant release (<2 h). Additionally, developed formulation exhibited an improved biodisponibility (25.1±0.2 h) in comparison to the commercially available formulation (1.7±0.1 h). The Solid Tumor Carcinoma model in Swiss albino mice revealed that the optimized formulation (based on gellan gum) showed better tumor reduction (85.7±1.2%) and lower toxicity as compared to the commercial formulation (77.3±1.3%). Pharmacokinetic and biodistribution studies demonstrated 3 to 4 times higher localization of drug in tumors. Our findings suggested that injectable gellan gum-based in situ hydrogel formulation can be an effective delivery system for DTX with enhanced solubility, reduced toxicity, and better targeting to the tumors for improved therapeutics.
Graphical abstract










Similar content being viewed by others
References
DE BREE EE, Katsougkri D, Polioudaki H, Tsangaridou E, Michelakis D, Zoras O, et al. Hyperthermia during intraperitoneal chemotherapy with paclitaxel or docetaxel for ovarian cancer: is there any benefit? Anticancer Res. 2020;40(12):6769–80.
Tang X, Wang G, Shi R, Jiang K, Meng L, Ren H, et al. Enhanced tolerance and antitumor efficacy by docetaxel-loaded albumin nanoparticles. Drug Deliv. 2016;23(8):2686–96.
EUROPEAN MEDICINES AGENCY Information for the package leaflet regarding 5 polysorbates used as excipients in medicinal products for 6 human use https://www.ema.europa.eu/en/documents/scientific-guideline/draft-information-package-leaflet-regarding-polysorbates-used-excipients-medicinal-products-human_en.pdf. Accessed on 21/10/2020.
Bajaj G, Kim MR, Mohammed SI, Yeo Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J Control Release. 2012;158(3):386–92.
Fan DY, Liu Z, Tian Y. Injectable hydrogels for localized cancer therapy. Front chem. 2019;7:675.
Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–52.
Vasir JK, Labhasetwar V. Targeted drug delivery in cancer therapy. Tech Canc Res & Treat. 2005;4(4):363–74.
LABEL FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf. Accessed on 08/12/2020.
National Drug Code List DOCETAXEL (https://ndclist.com/ndc/16729-267) Accessed on 15/10/2020.
Zahedi P, De Souza R, Piquette-Miller M, Allen C. Docetaxel distribution following intraperitoneal administration in mice. J Pharm Pharmaceutical Sci. 2011;14(1):90–9.
Cho JK, Hong JM, Han T, Yang HK, Song SC. Injectable and biodegradable poly (organophosphazene) hydrogel as a delivery system of docetaxel for cancer treatment. J Drug Target. 2013;21(6):564–73.
Fan R, Tong A, Li X, Gao X, Mei L, Zhou L, et al. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int J Nanomedicine. 2015;10:7291.
Gao L, Wang X, Ma J, Hao D, Wei P, Zhou L, et al. Evaluation of TPGS-modified thermo-sensitive Pluronic PF127 hydrogel as a potential carrier to reverse the resistance of P-gp-overexpressing SMMC-7721 cell lines. Colloids Surf B: Biointerfaces. 2016;140:307–16.
Mahajan M, Utreja P, Kumar Jain S. Paclitaxel loaded nanoliposomes in thermosensitive hydrogel: a dual approach for sustained and localized delivery. Anti-Cancer Agents Med. Chem. (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2016; 16(3): 365-376.
de Oliveira Cardoso VM, Cury BS, Evangelista RC, Gremião MP. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J Mech Behave Biomed Mat. 2017;65:317–33.
Tang J, Lelievre J, Ma T, Zeng Y. Polymer and ion concentration effects on gellan gel strength and strain. J Food Sci. 1994;59(1):216–20.
D’Arrigo G, Navarro G, Di Meo C, Matricardi P, Torchilin V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: a multi-drug delivery system for a combination therapy in cancer treatment. Eur J Pharm Biopharm. 2014;87(1):208–16.
Posadowska U, Brzychczy-Włoch M, Drożdż A, Krok-Borkowicz M, Włodarczyk-Biegun M, Dobrzyński P, et al. Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Exp Op drug deliv. 2016;13(5):613–20.
Gupta H, Velpandian T, Jain S. Ion-and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18(7):499–505.
Gong Y, Wang C, Lai RC, Su K, Zhang F, Wang DA. An improved injectable polysaccharide hydrogel: modified gellan gum for long-term cartilage regeneration in vitro. J Mater Chem. 2009;19(14):1968–77.
Singh A, Thakur S, Singh H, Singh H, Kaur S, Kaur S, Dudi R, Mondhe DM, Jain SK. Novel Vitamin E TPGS based docetaxel nanovesicle formulation for its safe and effective parenteral delivery: toxicological, pharmacokinetic and pharmacodynamic evaluation. 2020; J Lipo Res. 1-38.
Pereira GG, Dimer FA, Guterres SS, Kechinski CP, Granada JE, Cardozo NS. Formulation and characterization of poloxamer 407®: thermoreversible gel containing polymeric microparticles and hyaluronic acid. Química Nova. 2013;36(8):1121–5.
Labhasetwar V, Mohan MS, Dorle AK. A study on zeta potential and dielectric constant of liposomes. J Microencapsul. 1994;11(6):663–8.
Rao BM, Chakraborty A, Srinivasu MK, Devi ML, Kumar PR, Chandrasekhar KB, et al. A stability-indicating HPLC assay method for docetaxel. J Pharm Biomed Anal. 2006;41(2):676–81.
Panwar P, Pandey B, Lakhera PC, Singh KP. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int J Nanomedicine. 2010;5:101.
Ganji F, Abdekhodaie MJ, SA, AR. Gelation time and degradation rate of chitosan-based injectable hydrogel. J Sol-Gel Sci Tech. 2007;42(1):47–53.
Hurler J, Engesland A, Poorahmary Kermany B, Škalko-Basnet N. Improved texture analysis for hydrogel characterization: gel cohesiveness, adhesiveness, and hardness. J Appl Polym Sci. 2012;125(1):180–8.
Liu X, Gan H, Hu C, Sun W, Zhu X, Meng Z, et al. Silver sulfadiazine nanosuspension-loaded thermosensitive hydrogel as a topical antibacterial agent. Int J Nanomedicine. 2019;14:289.
Singh H, Kumar C, Singh N, Paul S, Jain SK. Nanoencapsulation of docosahexaenoic acid (DHA) using a combination of food grade polymeric wall materials and its application for improvement in bioavailability and oxidative stability. Food Funct. 2018;9(4):2213–27.
Thakur S, Singh H, Singh A, Kaur S, Sharma A, Singh SK, Kaur G, Jain SK. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: a dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J Drug Deliv Sci Tech. 2020; 101817.
Material Safety Data Sheet DOCETAXEL (https://pfe-pfizercom-d8- prod.s3.amazonaws.com/products/material_safety_data/Docetaxel_Injection_1-March-2016.pdf) Accessed on 15/10/2020.
Yılmaz S, Tokpınar A, Avnioğlu S Experimental cancer models
Rahman MS, Alam MB, Choi YH, Yoo JC. Anticancer activity and antioxidant potential of Aponogeton undulatus against Ehrlich ascites carcinoma cells in Swiss albino mice. Oncol Lett. 2017;14(3):3169–76.
Utreja P, Jain S, Tiwary AK. Evaluation of biosafety and intracellular uptake of Cremophor EL free paclitaxel elastic liposomal formulation. Drug Deliv. 2012;19(1):11–20.
Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003;10(3):185–91.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nature Rev Mat. 2016;1(12):1–7.
Hammadi NI, Abba Y, Hezmee MN, Razak IS, Jaji AZ, Isa T, et al. Formulation of a sustained release docetaxel loaded cockle shell-derived calcium carbonate nanoparticles against breast cancer. Pharm Res. 2017;34(6):1193–203.
Acknowledgements
Authors are thankful to the Department of Science of Technology (DST), New Delhi, India for providing financial support [Sanction no. EMR/2017/003648]. We are grateful to Government Medical College, Amritsar, Punjab, India for providing a facility for histopathology and IIIM, Jammu for cell line analysis and anticancer activity. Authors are also grateful to University Grant Commission (UGC), New Delhi to provide grants in aid to Guru Nanak Dev University, Amritsar under Rashtriya Uchchatar Shiksha Abhiyan (RUSA 2.0) Component 4.0 scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Thakur, S., Singh, N. et al. Novel Gellan Gum-Based In Situ Nanovesicle Formulation of Docetaxel for Its Localized Delivery Using Depot Formation. AAPS PharmSciTech 22, 165 (2021). https://doi.org/10.1208/s12249-021-02033-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02033-7