Abstract
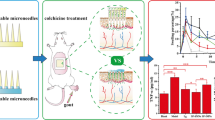
Salmon calcitonin (sCT) is a polypeptide drug, possessing the ability to inhibit osteoclast-mediated bone resorption. Just like other bioactive macromolecules, sCT is generally administered to the patients by either injection for poor compliance or through nasal spray for low bioavailability, which limits its use as therapeutic drugs. In the present study, to overcome the limitations of the conventional routes, two new dissolving microneedle arrays (DMNAs) based on transdermal sCT delivery systems were developed, namely sCT-DMNA-1 (sCT/Dex/K90E) and sCT-DMNA-2 (sCT/Dex-Tre/K90E) with the same dimension, meeting the requirements of suitable mechanical properties. An accurate and reliable method was established to determine the needle drug loading proportion in sCT-DMNAs. The stability study exhibited that the addition of trehalose could improve the stability of sCT in DMNA under high temperature and humidity. Further, in vivo pharmacodynamic study revealed that DMNA patch could significantly enhanced relative bioavailability to approximately 70%, and the addition of trehalose was found to be beneficial for sCT transdermal delivery. Therefore, sCT-DMNA is expected to replace traditional dosage form, providing a secure, efficient, and low-pain therapeutic strategy for bone disorders.





Similar content being viewed by others
References
Huang CLH, Sun L, Moonga BS, Zaidi M. Molecular physiology and pharmacology of calcitonin. Cell Mol Biol (Noisy-le-grand). 2006;52(3):33–43.
Naot D, Musson DS, Cornish J. The activity of peptides of the calcitonin family in bone. Physiol Rev. 2019;99(1):781–805. https://doi.org/10.1152/physrev.00066.2017.
Bandeira L, Lewiecki EM, Bilezikian JP. Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2016;12(6):681–9. https://doi.org/10.1080/17425255.2016.1175436.
Allison SL, Davis KW. Calcitonin stewardship strategies. J Pharm Pract. 2019;32:584–5. https://doi.org/10.1177/0897190018770052.
Ito A, Yoshimura M. Mechanisms of the analgesic effect of calcitonin on chronic pain by alteration of receptor or channel expression. Mol Pain. 2017;13:1744806917720316. https://doi.org/10.1177/1744806917720316.
Okabe K, Okamoto F, Kajiya H. Odontoclasts and calcitonin. Clin Calcium. 2012;22:19–26 CliCa12011926 (DOI Not Found).
Bajracharya R, Song JG, Back SY, Han HK. Recent advancements in non-invasive formulations for protein drug delivery. Comput Struct Biotechnol J. 2019;17:1290–308. https://doi.org/10.1016/j.csbj.2019.09.004.
Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16:153–7. https://doi.org/10.1038/nbt0298-153.
Torres-Lugo M, Peppas NA. Transmucosal delivery systems for calcitonin: a review. Biomaterials. 2000;21:1191–6. https://doi.org/10.1016/S0142-9612(00)00011-9.
Sun L, Le Z, He S, Liu J, Liu L, Leong KW, et al. Flash fabrication of orally targeted nanocomplexes for improved transport of salmon calcitonin across the intestine. Mol Pharm. 2020;17:757–68. https://doi.org/10.1021/acs.molpharmaceut.9b00827.
Liu L, Yang H, Lou Y, Wu JY, Miao J, Lu XY, et al. Enhancement of oral bioavailability of salmon calcitonin through chitosan-modified, dual drug-loaded nanoparticles. Int J Pharm. 2019;557:170–7. https://doi.org/10.1016/j.ijpharm.2018.12.053.
Onishi H, Tokuyasu A. Preparation and evaluation of enteric-coated chitosan derivative-based microparticles loaded with salmon calcitonin as an oral delivery system. Int J Mol Sci. 2016;17(9):1546. https://doi.org/10.3390/ijms17091546.
Asafo-Adje TA, Chen AJ, Najarzadeh A, Puleo DA. Advances in controlled drug delivery for treatment of osteoporosis. Curr Osteoporos Rep. 2016;14(5):226–38. https://doi.org/10.1007/s11914-016-0321-4.
Pontiroli AE. Peptide hormones: review of current and emerging uses by nasal delivery. Adv Drug Deliv Rev. 1998;29:81–7. https://doi.org/10.1016/S0169-409X(97)00062-8.
Manosroi J, Lohcharoenkal W, Götz F, Werner RG, Manosroi W, Manosroi A. Transdermal absorption and stability enhancement of salmon calcitonin by Tat peptide. Drug Dev Ind Pharm. 2013;39(4):520–5. https://doi.org/10.3109/03639045.2012.684388.
Amaro MI, Tewes F, Gobbo O, Tajber L, Corrigan OI, Ehrhardt C, et al. Formulation, stability and pharmacokinetics of sugar-based salmon calcitonin-loaded nanoporous/nanoparticulate microparticles (NPMPs) for inhalation. Int J Pharm. 2015;483(1–2):6–18. https://doi.org/10.1016/j.ijpharm.2015.02.003.
Tas C, Mansoor S, Kalluri H, Zarnitsyn VG, Choi SO, Banga AK, et al. Delivery of salmon calcitonin using a microneedle patch. Int J Pharm. 2012;423(2):257–63. https://doi.org/10.1016/j.ijpharm.2011.11.046.
Vemulapalli V, Bai Y, Kalluri H, Herwadkar A, Kim H, Davis SP, et al. In vivo Iontophoretic delivery of salmon calcitonin across microporated skin. J Pharm Sci. 2012;101(8):2861–9. https://doi.org/10.1002/jps.23222.
Gomaa YA, Garland MJ, McInnes F, El-K LK, Wilson C, Donnelly RF. Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium. Eur J Pharm Biopharm. 2012;82(2):299–307. https://doi.org/10.1016/j.ejpb.2012.07.008.
Wang Q, Yao G, Dong P, Gong Z, Li G, Zhang K, et al. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur J Pharm Sci. 2015;66:148–56. https://doi.org/10.1016/j.ejps.2014.09.011.
Yao G, Quan G, Lin S, Peng T, Wang Q, Ran H, et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: in vitro and in vivo characterization. Int J Pharm. 2017;534:378–86. https://doi.org/10.1016/j.ijpharm.2017.10.035.
Herwadkar A, Banga AK. Peptide and protein transdermal drug delivery. Drug Discov Today Technol. 2011;9(2):e71–e174. https://doi.org/10.1016/j.ddtec.2011.11.007.
Cetin M, Youn YS, Capan Y, Lee KC. Preparation and characterization of salmon calcitonin-biotin conjugates. AAPS PharmSciTech. 2008;9(4):1191–7. https://doi.org/10.1208/s12249-008-9165-2.
Sun P, Zhang X, Chen Y, Zang X. Application of the yeast-surface-display system for orally administered salmon calcitonin and safety assessment. Biotechnol Prog. 2010;26(4):968–74. https://doi.org/10.1002/btpr.413.
Karsdal MA, Byrjalsen I, Henriksen K, Riis BJ, Christiansen C. A pharmacokinetic and pharmacodynamic comparison of synthetic and recombinant oral salmon calcitonin. J Clin Pharmacol. 2009;49(2):229–34. https://doi.org/10.1177/0091270008329552.
Millest AJ, Evans JR, Young JJ, Johnstone D. Sustained release of salmon calcitonin in vivo from lactide: glycolide copolymer depots. Calcif Tissue Int. 1993;52(5):361–4. https://doi.org/10.1007/BF00310200.
Feng J, Fitz Y, Li Y, Fernandez M, Cortes PI., Wang D, et al. Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. J Vis Exp. 2015;30(95). https://doi.org/10.3791/51881.
Hartmann AE, Lewis LR. Evaluation of the ASTRA o-cresolphthalein complexone calcium method. Am J Clin Pathol. 1984;82(2):182–7. https://doi.org/10.1093/ajcp/82.2.182.
Cohen SA, Sideman L. Modification of the o-cresolphthalein complexone method for determining calcium. Clin Chem. 1979;25(8):1519–20.
Zhang T, Heimbach T, Lin W, Zhang J, He H. Prospective predictions of human pharmacokinetics for eighteen compounds. J Pharm Sci. 2015;104(9):2795–806. https://doi.org/10.1002/jps.24373.
Lock JY, Carlson TL, Carrier RL. Mucus models to evaluate the diffusion of drugs and particles. Adv Drug Deliv Rev. 2018;124(15):34–49. https://doi.org/10.1016/j.addr.2017.11.001.
Okamura E. Solution NMR to quantify mobility in membranes: diffusion, protrusion, and drug transport processes. Chem Pharm Bull. 2019;67(4):308–15. https://doi.org/10.1248/cpb.c18-00946.
Crowe LM. Lessons from nature: the role of sugars in anhydrobiosis. Comp Biochem Physiol. 2002;131A:505–13. https://doi.org/10.1016/S1095-6433(01)00503-7.
Crowe JH, Hoekstra FA, Crowe L. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–99. https://doi.org/10.1146/annurev.ph.54.030192.003051.
Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multi functional molecule. Glycobiology. 2003;13:17–27. https://doi.org/10.1093/glycob/cwg047.
Oliveira IP, Martínez L. The shift in urea orientation at protein surfaces at low pH is compatible with a direct mechanism of protein denaturation. Phys Chem Chem Phys. 2019;22(1):354–67. https://doi.org/10.1039/c9cp05196a.
Diamant S, Eliahu N, Rosenthal D, Goloubin P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem. 2001;276:39586–91. https://doi.org/10.1074/jbc.M103081200.
Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–15. https://doi.org/10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2.
Singer MA, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–48. https://doi.org/10.1016/s1097-2765(00)80064-7.
Corradini D, Strekalova EG, Stanley HE, Gallo P. Microscopic mechanism of protein cryopreservation in an aqueous solution with trehalose. Sci Rep. 2013;3:1218. https://doi.org/10.1038/srep01218.
Laskowska E, Kuczyńska-Wiśnik D. New insight into the mechanisms protecting bacteria during desiccation. Curr Genet. 2020;66(2):313–8. https://doi.org/10.1007/s00294-019-01036-z.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81502994), Key Projects of Outstanding Young Talents Support Program in universities of Anhui Province (Grant No. gxyqZD2020026), and Natural Science Foundation of Anhui Province (Grant No. 1608085QH179).
Author information
Authors and Affiliations
Contributions
Lu Zhang: conceptualization, methodology, software, investigation, writing—original draft.
Yingying Li: validation, formal analysis, visualization, software.
Fang Wei: validation, formal analysis, visualization.
Hang Liu: resources, writing—review and editing, supervision, data curation.
Yushuai Wang: resources, writing—review and editing, supervision, data curation.
Weiman Zhao: resources, writing—review and editing, supervision.
Zhiyong Dong: writing—review and editing.
Tao Ma: writing—review and editing.
Qingqing Wang: writing—review and editing.
Corresponding author
Ethics declarations
All operations were approved by the Animal Ethical Committee of Sun Yat-sen University and were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Conflict of Interest
The authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Li, Y., Wei, F. et al. Transdermal Delivery of Salmon Calcitonin Using a Dissolving Microneedle Array: Characterization, Stability, and In vivo Pharmacodynamics. AAPS PharmSciTech 22, 1 (2021). https://doi.org/10.1208/s12249-020-01865-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01865-z