Abstract
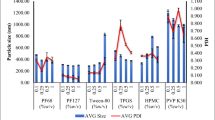
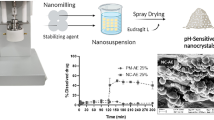
Puerarin is widely used as a therapeutic agent to cardiovascular diseases in clinics in China through intravenous administration, which could elicit adverse drug reactions caused by cosolvents, hindering its application in clinics. Therefore, the development of oral dosage is urgently needed. In our previous studies, we proved that the bioavailability of puerarin increased as particle sizes of nanocrystals decreased; however, we have not optimized the best process parameters for nanocrystals. In this study, we aim to fabricate fine nanocrystals (with smallest particle size) by Box–Behnken design and study the intestinal permeability of puerarin and its nanocrystals via employing everted gut sac model and in situ perfusion model. The results showed that the Box–Behnken design could be used to optimize the producing parameters of puerarin nanocrystals, and the particle sizes of fine nanocrystals were about 20 nm. Results of everted gut sacs showed that the polyvinylpyrrolidone (PVP) and verapamil had no influence on the absorption of puerarin and nanocrystals, and the nanocrystals could increase the Papp of puerarin for 2.2-, 2.9-, and 2.9-folds, respectively, in duodenum, jejunum, and ileum. Enhanced Ka and Peff were observed on the nanocrystal group, compared with puerarin, and PVP and verapamil had no influence on the absorption of nanocrystals, while the absorption of puerarin was influenced by P-gp efflux. Combining the results mentioned above, we can conclude that the Box–Behnken design benefits the optimization for preparation of nanocrystals, and the nanocrystals could enhance the intestinal absorption of puerarin by enhanced permeability and inhibited P-gp efflux.









Similar content being viewed by others
References
Chen R, Xue J, Xie ML. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-β1 expression. J Nutr Biochem. 2012;23(9):1080–5.
Chen XH, Tian DQ, Wei LR. Literature analysis of adverse reactions induced by puerarin injection. Practical Pharmacy and Clinical Remedies. 2010;13(4):293–5.
Gao L, Liu GY, Ma JL, Wang XQ, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30.
Keck CM, Kobierski S, Mauludin R, Müller RH. Second generation of drug nanocrystals for delivery of poorly soluble drugs: smartcrystals technology. Dosis. 2008;24(2):124–8.
Lu Y, Chen Y, Gemeinhart RA, Wu W, Li T. Developing nanocrystals for cancer treatment. Nanomedicine-UK. 2015;10(16):2537–52.
Lu Y, Li Y, Wu W. 2016. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B 2016;6(2):106–113.
Wang GD, Mallet FP, Ricard F, Heng JYY. Pharmaceutical nanocrystals. Curr Opin Chem Eng. 2012;1(2):102–7.
Yue PF, Xiao MS, Xie YB, Ma YM, Guan YM, Wu ZF, et al. The roles of vitrification of stabilizers/matrix formers for the redispersibility of drug nanocrystals after solidification: a case study. AAPS PharmSciTech. 2016;17(6):1274–84.
Xie YB, Ma YQ, Xu JN, Liu Y, Yue PF, Zheng Q, et al. Panax notoginseng saponins as a novel nature stabilizer for poorly soluble drug nanocrystals: a case study with baicalein. Molecules. 2016;21(9):1149.
Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23(3):534–47.
Toziopoulou F, Malamatari M, Nikolakakisa I, Kachrimanis K. Production of aprepitant nanocrystals by wet media milling and subsequent solidification. Int J Pharm. 2017;533(2):324–34.
Gao L, Zhang DR, Chen MH. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res. 2008;10(5):845–62.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Tu LX, Yi YN, Wu W, Hu FQ, Hu KL, Feng JF. Effects of particle size on the pharmacokinetics of puerarin nanocrystals and microcrystals after oral administration to rat. Int J Pharm. 2013;458(1):135–40.
Yi YN, Tu LX, Hu KL, Wu W, Feng JF. The construction of puerarin nanocrystals and its pharmacokinetic and in vivo–in vitro correlation (IVIVC) studies on beagle dog. Colloid Suface B. 2015;133(1):164–70.
Roger E, Lagarce F, Garcion E, Benoit JP. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine-UK. 2010;5(2):287–306.
Yu Q, Wu XY, Zhu QG, Wu W, Chen ZJ, Li Y, et al. Enhanced transdermal delivery of meloxicam by nanocrystals: preparation, in vitro and in vivo evaluation. Asian J Pharm Sci. 2018;13(6):518–26.
Xie YK, Shi BK, Xia F, Qi JP, Dong XC, Zhao WL, et al. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J Control Release. 2017;270(28):65–75.
Lu Y, Qi JP, Dong XC, Zhao WL, Wu W. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22(4):744–50.
Ma YQ, Yang Y, Xie J, Yue PF, Yang M. Novel nanocrystal-based solid dispersion with high drug loading, enhanced dissolution, and bioavailability of andrographolide. Int J Nanomedicine. 2018;13:3763–79.
Kalam MA, Khan AA, Khan S, Almalik A, Alshamsan A. Optimizing indomethacin-loaded chitosan nanoparticle size encapsulation, and release using Box–Behnken experimental design. Int J Biol Macromol. 2016;87:329–40.
Xi J, Guo R. Interactions of puerarin with micelles: pKa shifts and thermodynamics. J Solut Chem. 2008;37:107–18.
Sa CL, Lv H, Ba YY, Sun JN, Shi RB. The effects of notoginsenoside R1 on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol. 2012;142(1):136–43.
Vora AK, Londhe VY, Pandita NS. Preparation and characterization of standardized pomegranate extract-phospholipid complex as an effective drug delivery tool. J Adv Pharm Technol Res. 2015;6(2):75–80.
Liu HL, Tu LX, Zhou YX, Dang ZF, Wang LT, Du JF, et al. Improved bioavailability and antitumor effect of docetaxel by TPGS modified proniosomes: in vitro and in vivo evaluations. Sci Rep-UK. 2017;7:43372.
Tambe A, Mokashi P, Pandita N. Ex-vivo intestinal absorption study of boswellic acid, cyclodextrin complexes and poloxamer solid dispersions using everted gut sac technique. J Pharmaceut Biomed. 2019;167(15):66–73.
Huang S, Zhang Q, Li H, Sun YQ, Cheng G, Zou MJ, et al. Increased bioavailability of efonidipine hydrochloride nanosuspensions by the wet-milling method. Eur J Pharm Biopharm. 2018;130:108–14.
Dahan A, West BT, Amidon GL. Segmental-dependent membrane permeability along the intestine following oral drug administration: evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur J Pharm Sci. 2009;36(2–3):320–9.
Li HL, Zhao XB, Ma YK, Zhai GX, Li LB, Lou HX. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44.
Greeshma VP, Vaibhav BP, Abhishek P, Sadhana JR. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40(1):80–91.
Sun MH, Zhai XZ, Xue KW, Hu L, Yang XL, Li G, et al. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci. 2011;43(3):132–40.
Dahlgeren D, Roos C, Sjŏgren E, Lennernăs H. Direct in vivo human intestinal permeability (Peff) determined with different clinical perfusion and intubation methods. J Pharm Sci-US. 2015;104(9):2702–26.
Mohd Y, Udai VSS, Iti C, Praveen KG, Alok PS, Dinesh P, et al. Solid lipid nanoparticles for nose to brain delivery of donepezil: formulation, optimization by Box-Behnken design, in vitro and in vivo evaluation. Artif Cell Nanomed B. 2018;46(8):1838–51.
Hao JF, Fang XS, Zhou YF, Wang JZ, Guo FG, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683–92.
Kumar S, Narayan R, Ahammed V, Nayak Y, Naha A, Nayak UY. Development of ritonavir solid lipid nanoparticles by Box Behnken design for intestinal lymphatic targeting. J Drug Deliv Sci Tec. 2018;44:181–9.
Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: preparation by a size-reduction technique. Int J Pharm. 1998;160(2):229–37.
Skrdla PJ, Floyd PD, Dell’Orco PC. Predicting the solubility enhancement of amorphous drugs and related phenomena using basic thermodynamic principles and semi-empirical kinetic models. Int J Pharm. 2019;567:118465.
Yaňez F, Concheiro A, Alvarez-Lorenzo C. Macromolecule release and smoothness of semi-interpenetrating PVP–pHEMA networks for comfortable soft contact lenses. Eur J Pharm Biopharm. 2008;69:1094–03.
Zhou Y, Song X, Dong G. Effects of verapamil on the pharmacokinetics of puerarin in rats. Xenobiotica. 2018;49(10):1178–82.
Yi T, Tang DD, Wang F, Zhang JQ, Zhang J, Wang JR, et al. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Deliv. 2017;24(1):422–9.
Ballent M, Lifschitz A, Virkel G, Sallovitz J, Lanusse C. Modulation of the P-glycoprotein-mediated intestinal secretion of ivermectin: in vitro and in vivo assessments. Drug Metab Dispos. 2006;34(3):457–63.
Li M, Si LQ, Pan HP, Rabba AK, Yan F, Qiu J, et al. Excipients enhance intestinal absorption of ganciclovir by P-gp inhibition: assessed in vitro by everted gut sac and in situ by improved intestinal perfusion. Int J Pharm. 2011;403(1–2):37–45.
Ueda K, Iwai T, Sunazuka Y, Chen Z, Kato N, Higashi K, et al. Effect of molecular weight of hypromellose on mucin diffusion and oral absorption behavior of fenofibrate nanocrystal. Int J Pharm. 2019;564:39–47.
Yeh T, Hsu L, Tseng M, Lee P, Sonjae K, Ho Y, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–73.
Sharma S, Verma A, Pandey G, Mittapelly N, Mishra PR. Investigating the role of Pluronic-g-cationic polyelectrolyte as functional stabilizer for nanocrystals: impact on paclitaxel oral bioavailability and tumor growth. Acta Biomater. 2015;26:169–83.
Miao X, Li Y, Wang X, Lee S, Zheng Y. Transport mechanism of coumarin 6 nanocrystals with two particle sizes in MDCKII monolayer and larval zebrafish. ACS Appl Mater Interfaces. 2016;8(20):12620–30.
Liu DD, Xu HM, Tian BC, Yuan K, Pan H, Ma SL, et al. Fabrication of carvedilol nanosuspensions through the anti-solvent precipitation–ultrasonication method for the improvement of dissolution rate and oral bioavailability. AAPS PharmSciTech. 2012;13(1):295–04.
Deng FY, Zhang H, Wang X, Zhang Y, Hu HX, Song SY, et al. Transmembrane pathways and mechanisms of rod-like paclitaxel nanocrystals through MDCK polarized monolayer. ACS Appl Mater Interfaces. 2017;9(7):5803–16.
Guo MR, Wei MD, Li W, Guo MC, Guo CL, Ma MC, et al. Impacts of particle shapes on the oral delivery of drug nanocrystals: mucus permeation, transepithelial transport and bioavailability. J Control Release. 2019;307:64–75.
Xie YK, Shi BK, Xia F, Qi JP, Dong XC, Zhao WL, et al. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J Control Release. 2017;270:65–75.
Funding
This work was supported by the National Natural Science Foundation of China (81960717, 81573623), the Natural Science Foundation of Jiangxi province (20192BAB215057), and the “Double First-Class” discipline project of Jiangxi Province (JXSYLXK-ZHYA0015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Statement
All procedures were approved by the Animal Research Ethics Committee, Jiangxi University of Traditional Chinese Medicine.
Conflict of Interest
The authors declare there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Cheng, M., Yuan, F., Liu, J. et al. Fabrication of Fine Puerarin Nanocrystals by Box–Behnken Design to Enhance Intestinal Absorption. AAPS PharmSciTech 21, 90 (2020). https://doi.org/10.1208/s12249-019-1616-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1616-4