Abstract
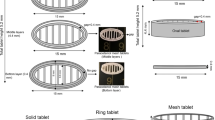
Thermal extrusion (TE) 3D printing is a thermoplastic semisolid-based rapid prototyping process, which is capable of building complex structures. The aim of this study was to manufacture rapid-release puerarin tablets without solvent through TE 3D printing. Novel rapid-release tablets were fabricated with polyethylene glycol (PEG 4000) as the carrier at appropriate puerarin/PEG 4000 ratios, assessed through differential scanning calorimetry (DSC), solubility, and dissolution tests. The novel structures of 3D-printed tablets with five different values were formed by printing paths, which established a flexible way of adjusting in vitro drug release. An obvious acceleration (85% of cumulative release about 7.5 min at the soonest) was observed for the tablets with internal structural design. It was inferred that puerarin formed simple eutectic mixtures with PEG 4000 and that puerarin dispersed into the carrier based on DSC and X-Ray powder diffraction (XRD). This highlights the combined advantage of PEG as a soluble polymer with TE 3D printing and provides a suitable system for rapid puerarin release.





Similar content being viewed by others
References
Park S, Jun S. Statistical technology analysis for competitive sustainability of three dimensional printing. Sustainability. 2017;9(7):1142.
Preis M, Öblom H. 3D-printed drugs for children—are we ready yet? AAPS PharmSciTech. 2017;18(2):303–8.
Melocchi A, Parietti F, Maccagnan S, Ortenzi MA, Antenucci S, Briatico-Vangosa F, et al. Industrial development of a 3D-printed nutraceutical delivery platform in the form of a multicompartment HPC capsule. AAPS PharmSciTech. 2018;19(8):3343–54.
Nukala PK, Palekar S, Patki M, Patel K. Abuse deterrent immediate release egg-shaped tablet (egglets) using 3D printing technology: quality by design to optimize drug release and extraction. AAPS PharmSciTech. 2019;20(2):80.
Öblom H, Zhang J, Pimparade M, Speer I, Preis M, Repka M, et al. 3D-printed isoniazid tablets for the treatment and prevention of tuberculosis—personalized dosing and drug release. AAPS PharmSciTech. 2019;20(2):52.
Korte C, Quodbach J. 3D-printed network structures as controlled-release drug delivery systems: dose adjustment, API release analysis and prediction. AAPS PharmSciTech. 2018;19(8):3333–42.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Sadia M, Arafat B, Ahmed W, Forbes RE, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2017;269:1–32.
Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261:207–15.
Li Q, Guan X, Cui M, Zhu Z, Chen K, Wen H, et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int J Pharm. 2018;535(1-2).
Martinez PR, Goyanes A, Basit AW, Gaisford S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech. 2018;19(8):3355–61.
Khaled SA, Alexander MR, Irvine DJ, Wildman RD, Wallace MJ, Sharpe S, et al. Extrusion 3D printing of paracetamol tablets from a single formulation with tunable release profiles through control of tablet geometry. AAPS PharmSciTech. 2018;19(8):3403–13.
Wen H, He B, Wang H, Chen F, Li P, Cui M, et al. Structure-based gastro-retentive and controlled-release drug delivery with novel 3D printing. AAPS PharmSciTech. 2019;20(2):68.
Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Technol. 2015;30:360–7.
Sadia M, Sośnicka A, Arafat B, Isreb A, Ahmed W, Kelarakis A, et al. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int J Pharm. 2016;513(1):659–68.
Alvaro G, Buanz ABM, Hatton GB, Simon G, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–62.
Okwuosa TC, Stefaniak D, Arafat B, Isreb A, Wan KW, Alhnan MA. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm Res. 2016;33(11):1–9.
Kempin W, Domsta V, Grathoff G, Brecht I, Semmling B, Tillmann S, et al. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm Res. 2018;35(6):124.
Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33(8):1817–32.
Guyot M, Fawaz F, Bildet J, Bonini F, Lagueny A-M. Physicochemical characterization and dissolution of norfloxacin/cyclodextrin inclusion compounds and PEG solid dispersions. Int J Pharm. 1995;123(1):53–63.
Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm. 1995;126(1–2):155–60.
Doshi DH, Ravis WR, Betageri GV. Carbamazepine and polyethylene glycol solid dispersions: preparation, in vitro dissolution, and characterization. Drug Dev Commun. 2008;23(12):1167–76.
Zerrouk N, Chemtob C, Arnaud P, Toscani S, Dugue J. In vitro and in vivo evaluation of carbamazepine-PEG 6000 solid dispersions. Int J Pharm. 2001;225(1):49–62.
Law D, Schmitt EA, Marsh KC, Everitt EA, Wang W, Fort JJ, et al. Ritonavir-PEG 8000 amorphous solid dispersions: in vitro and in vivo evaluations. J Pharm Sci. 2004;93(3):563–70.
Liu C, Desai KGH. Characteristics of rofecoxib-polyethylene glycol 4000 solid dispersions and tablets based on solid dispersions. Pharm Dev Technol. 2005;10(4):467–77.
Chia-Chi C, Wen-Hsiung C. Impact effects of puerarin on mouse embryonic development. Reprod Toxicol. 2009;28(4):530–5.
Choi YM, Jun HJ, Dawson K, Rodriguez RL, Mi RR, Jun J, et al. Effects of the isoflavone puerarin and its glycosides on melanogenesis in B16 melanocytes. Eur Food Res Technol. 2010;231(1):75–83.
Luo CF, Yuan M, Chen MS, Liu SM, Zhu L, Huang BY, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410(1):138–44.
Jia S, Zhen M, Hui S, Xing D, Yi D, Du L. Different kinetics of puerarin in plasma of normal and depressed rats after oral administration of Chinese medicine TZ18. Tsinghua Sci Technol. 2007;12(4):394–9.
Zhang Y, Wang R, Wu J, Shen Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int J Pharm. 2012;427(2):337–44.
Hongfei W, An Z, Chuanhua L, Lei W. Examination of lymphatic transport of puerarin in unconscious lymph duct-cannulated rats after administration in microemulsion drug delivery systems. Eur J Pharm Sci. 2011;42(4):348–53.
Deshkar SS, Borde G, Kale R, Waghmare B, Thomas AB. Formulation of cilostazol spherical agglomerates by crystallo-co-agglomeration technique and optimization using design of experimentation. Int J Pharm Investig. 2017;7(4):164–73.
Shah VP, Tsong Y, Sathe P, Liu J-P. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15(6):889–96.
Karalis V, Magklara E, Shah VP, Macheras P. From drug delivery systems to drug release, dissolution, IVIVC, BCS, BDDCS, bioequivalence and biowaivers. Pharm Res. 2010;27(9):2018–29.
Costa P, Lobo JMS. Divisability of diltiazem matrix sustained-release tablets. Pharm Dev Technol. 2001;6(3):343–51.
Costa P. An alternative method to the evaluation of similarity factor in dissolution testing. Int J Pharm. 2001;220(1):77–83.
Wang Q, Yu D-G, Zhang L-L, Liu X-K, Deng Y-C, Zhao M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr Polym. 2017;174:617–25.
Huang W, Hou Y, Lu X, Gong Z, Yang Y, Lu X-J, et al. The process–property–performance relationship of medicated nanoparticles prepared by modified coaxial electrospraying. Pharmaceutics. 2019;11(5):226.
Huang W, Yang Y, Zhao B, Liang G, Liu S, Liu X-L, et al. Fast dissolving of ferulic acid via electrospun ternary amorphous composites produced by a coaxial process. Pharmaceutics. 2018;10(3):115.
Li J-J, Yang Y-Y, Yu D-G, Du Q, Yang X-L. Fast dissolving drug delivery membrane based on the ultra-thin shell of electrospun core-shell nanofibers. Eur J Pharm Sci. 2018;122:195–204.
Liu Z-P, Zhang L-L, Yang Y-Y, Wu D, Jiang G, Yu D-G. Preparing composite nanoparticles for immediate drug release by modifying electrohydrodynamic interfaces during electrospraying. Powder Technol. 2018;327:179–87.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7.
Shah JC, Chen JR, Chow D. Preformulation study of etoposide: II. Increased solubility and dissolution rate by solid-solid dispersions. Int J Pharm. 1995;113(1):103–11.
Matsuda Y, Tatsumi E. Physicochemical characterization of furosemide modifications. Int J Pharm. 1990;60(1):11–26.
Khattab IS, Nada A, Zaghloul AA. Physicochemical characterization of gliclazide-macrogol solid dispersion and tablets based on optimized dispersion. Drug Dev Ind Pharm. 2010;36(8):893–902.
Acknowledgments
We are grateful for the instruments purchased from Jingxin Pharmaceutical Co., Ltd. (Zhejiang, China).
Funding
This work was supported by National Science and Technology Major Project which belongs to “The research on the key technology of new drug delivery system and industrialization of new projects” (No. 2017ZX09201-003-011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, P., Jia, H., Zhang, S. et al. Thermal Extrusion 3D Printing for the Fabrication of Puerarin Immediate-Release Tablets. AAPS PharmSciTech 21, 20 (2020). https://doi.org/10.1208/s12249-019-1538-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1538-1