Abstract
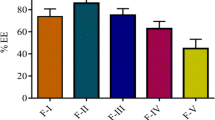
The objective of the present study was to develop a proliposomal formulation to increase the oral bioavailability of dronedarone hydrochloride (dronedarone HCl) by enhancing solubility, dissolution, and/or intestinal absorption. Proliposomes were prepared by using solvent evaporation method. In this process, different ratios of drug, phospholipids, such as soy phosphatidylcholine (SPC), Phospholipon 90H, hydrogenated egg phosphatidylcholine (HEPC), and dimyristoyl phosphatidylglycerol (DMPG), and cholesterol were used. Physical characterization and in vitro dissolution studies were evaluated for the prepared formulations. In vitro transport across the membrane was carried out using Caco-2 cells. Among all the formulations, the amount of drug released in dissolution was higher with DPF8 formulation (drug:DMPG Na:cholesterol:::1:2:0.2) compared to the pure drug. Also, Caco-2 cell permeability studies resulted in 2.6-fold increase in apparent permeability. Optimized formulation was evaluated in vivo in male Sprague–Dawley rats. After single oral administration of optimized formulation (DPF8), a relative bioavailability of 148.36% was achieved compared to the pure drug. Improved oral bioavailability of dronedarone could be provided by an optimized proliposomal formulation with enhanced solubility, permeability, and oral absorption.









Similar content being viewed by others
References
Gupta H, Bhandari D, Sharma A. Recent trends in oral drug delivery: a review. Recent Pat Drug Deliv Formul. 2009;3(2):162–73. https://doi.org/10.2174/187221109788452267.
Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci. 1993;82(10):979–87. https://doi.org/10.1002/jps.2600821002.
Bobbala SKR, Veerareddy PR. Formulation, evaluation, and pharmacokinetics of isradipine proliposomes for oral delivery. J Liposome Res. 2012;22(4):285–94. https://doi.org/10.3109/08982104.2012.697067.
Hiremath PS, Soppimath KS, Betageri GV. Proliposomes of exemestane for improved oral delivery: formulation and in vitro evaluation using PAMPA, Caco-2 and rat intestine. Int J Pharm. 2009;380(1):96–104. https://doi.org/10.1016/j.ijpharm.2009.07.008.
Christopher AL, Franco L, Beryl WD, Paul JF. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. https://doi.org/10.1016/S0169-409X(00)00129-0.
Chowdary KPR, Prakasarao KS. Individual and combined effects of cyclodextrins, poloxamer and PVP on the solubility and dissolution rate of BCS class II drugs. Asian J Chem. 2011;23(10):4520–4.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharmaceutics. 2012;1–10:195727. https://doi.org/10.5402/2012/195727.
Chowdary KPR, Prakasarao KS, Sirisha V. A factorial study on the effects of β cyclodextrin and poloxamer 407 on the solubility and dissolution rate of piroxicam. Int J Res Pharm Chem. 2011;1(3):601–5.
Aungst BJ, Saitoh H, Burcham DL, Huang S-M, Mousa SA, Hussain MA. Enhancement of the intestinal absorption of peptides and nonpeptides. J Control Release. 1996;41(1):19–31. https://doi.org/10.1016/0168-3659(96)01353-3.
Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, et al. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9(6):304–16. https://doi.org/10.1016/j.ajps.2014.05.005.
Porter CJ, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev. 2001;50(1):61–80. https://doi.org/10.1016/S0169-409X(01)00151-X.
Xu H, He L, Nie S, Guan J, Zhang X, Yang X, et al. Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. J Control Release. 2009;140(1):61–8. https://doi.org/10.1016/j.jconrel.2009.07.014.
Velpula A, Jukanti R, Janga KY, Veerareddy PR. Bioavailability enhancement of zaleplon via proliposomes: role of surface charge. Eur J Pharm Biopharm. 2012;80(2):347–57. https://doi.org/10.1016/j.ejpb.2011.10.010.
Payne NI, Timmins P, Ambrose CV, Ward MD, Ridgway F. Proliposomes: a novel solution to an old problem. J Pharm Sci. 1986;75(4):325–9. https://doi.org/10.1002/jps.2600750402.
Manjula D, Shabaraya AR, Somashekar S. Topical delivery of fenoprofen proliposomes: preparation, evaluation and in vitro release. Int J Pharma Sci Invent. 2014;3(8):6–12.
Agnihotri SA, Soppimath KS, Betageri GV. Controlled release application of multilamellar vesicles: a novel drug delivery approach. Drug Deliv. 2010;17(2):92–101. https://doi.org/10.3109/10717540903509027.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. https://doi.org/10.1007/s11095-004-9004-4.
Kazi M, Alamri R, Alanazi FK, Hussain MD. In vitro methods for in vitro-in vivo correlation (IVIVC) for poorly water soluble drugs: lipid based formulation perspective. Curr Drug Deliv. 2018;15:918–29. https://doi.org/10.2174/1567201815666180116090910.
Iram F, Ali S, Ahmad A, Khan SA, Husain A. A review on dronedarone: pharmacological, pharmacodynamic and pharmacokinetic profile. J Acute Dis. 2016;5(2):102–8. https://doi.org/10.1016/j.joad.2015.10.002.
Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357(10):987–99. https://doi.org/10.1056/NEJMoa054686.
Hussar DA. Prasugrel hydrochloride, dronedarone, and saxagliptin hydrochloride. J Am Pharm Assoc. 2009;49(6):832–6. https://doi.org/10.1331/JAPhA.2009.09544.
Chu C, Tong S-S, Xu Y, Wang L, Fu M, Ge Y-R, et al. Proliposomes for oral delivery of dehydrosilymarin: preparation and evaluation in vitro and in vivo. Acta Pharmacol Sin. 2011;32(7):973–80. https://doi.org/10.1038/aps.2011.25.
Arregui JR, Kovvasu SP, Betageri GV. Daptomycin proliposomes for oral delivery: formulation, characterization, and in vivo pharmacokinetics. AAPS PharmSciTech. 2018;19(4):1802–9. https://doi.org/10.1208/s12249-018-0989-0.
Nekkanti VK, Javier R, Wang Z, Betageri GV. Design, characterization, and in vivo pharmacokinetics of tacrolimus proliposomes. AAPS PharmSciTech. 2016;17(5):1019–29. https://doi.org/10.1208/s12249-015-0428-4.
Bermejo M, Avdeef RA, Nalda R, Ruell JA, Tsinman O, Gonzalez I, et al. PAMPA-A drug absorption in vitro model 7. Comparing rat in situ, Caco-2, and PAMPA permeability of fluoroquinolones. Eur J Pharm Sci. 2004;21(4):429–41. https://doi.org/10.1016/j.ejps.2003.10.009.
Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–37. https://doi.org/10.1016/S0167-7799(00)89017-4.
Potluri P, Betageri GV. Mixed-micellar proliposomal systems for enhanced oral delivery of progesterone. Drug Deliv. 2006;13(3):227–32. https://doi.org/10.1080/10717540500395007.
Söderlund T, Lehtonen JYA, Kinnunen PKJ. Interactions of cyclosporin A with phospholipid membranes: effect of cholesterol. Mol Pharmacol. 1999;55(1):32–8. https://doi.org/10.1124/mol.55.1.32.
Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci. 2009;106(10):3654–8. https://doi.org/10.1073/pnas.0809959106.
Balimane PV, Han YH, Chong S. Current industrial practices of assessing permeability and p-glycoprotein interaction. AAPS J. 2006;8(1):E1–13.
Lakeram M, Lockley D, Pendlington R, Forbes B. Optimization of the Caco-2 permeability assay using experimental design methodology. Pharm Res. 2008;25(7):1544–51. https://doi.org/10.1007/s11095-008-9556-9.
Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134(4):1031–49. https://doi.org/10.1083/jcb.134.4.1031.
Bathori G, Cervenak L, Karadi I. Caveolae—an alternative endocytotic pathway for targeted drug delivery. Crit Rev Ther Drug Carrier Syst. 2004;21(2):67–95. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v21.i2.10.
Huth US, Schubert R, Peschka SR. Investigating the uptake and intracellular fate of pH-sensitive liposomes by flow cytometry and spectral bio-imaging. J Control Release. 2006;110(3):490–504. https://doi.org/10.1016/j.jconrel.2005.10.018.
Acknowledgments
The authors express their sincere gratitude to the Western University of Health Sciences, Pomona, California, for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovvasu, S.P., Kunamaneni, P., Yeung, S. et al. Formulation of Dronedarone Hydrochloride-Loaded Proliposomes: In Vitro and In Vivo Evaluation Using Caco-2 and Rat Model. AAPS PharmSciTech 20, 226 (2019). https://doi.org/10.1208/s12249-019-1437-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1437-5