Abstract
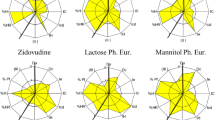
The SeDeM expert system is used to reveal direct compression (DC) suitability of the active ingredients and excipients in preformulation. In this study, the system was used to predict compressibility of rhodiola extract (RhE) and its mixture with excipients. The parameter index (IP), parameter profile index (IPP), and good compressibility index (IGC) of RhE mixtures with different fillers were investigated. The results showed that RhE and mixture with lactose or starch were not suitable for DC according to the values of IP, IPP, and IGC, which can be corrected by pregelatinized starch (P-STA). The quality of tablets corrected by P-STA all satisfied the USP monograph limit. The findings from this study showed that the system is a useful tool to predict DC suitability on the mixture of RhE and an excipient.





Similar content being viewed by others
References
Suñé Negre JM, Roig Carreras M, Fuster García R, Hernández Pérez C, Ruhí Roura R, García Montoya E, et al. Nueva metodología de preformulación galénica para la caracterización de ustancias en relación a su viabilidad para la compresión: diagrama SeDeM. Cien Technol Pharm. 2005;15(3):125–36.
Borges PF, Pérez-Lozano P, Suñé-Negre JM, Miñarro M, Manich A, Jo E, et al. The role of SeDeM for characterizing the active substance and polyvinyilpyrrolidone eliminating metastable forms in an oral lyophilizate—a preformulation study. PLoS One. 2018;13(4):e0196049. https://doi.org/10.1371/journal.pone.0196049.
Aguilar-Díaz JE, García-Montoya E, Suñe-Negre JM, Pérez-Lozano P, Miñarro M, Ticó JR. Predicting orally disintegrating tablets formulations of ibuprophen tablets: an application of the new SeDeM-ODT expert system. Eur J Pharm Biopharm. 2012;80(3):638–48.
Hamman H, Hamman J, Wessels A, Scholtz J, Steenekamp JH. Development of multiple-unit pellet system tablets by employing the SeDeM expert diagram system I: pellets with different sizes. Pharm Dev Technol. 2017;23(7):706–14.
Tadwee I, Shahi S. Formulation development of losartan potassium immediate release tablets and process optimization using SeDeM expert system. J Appl Pharm Sci. 2018;8(2):33–43.
Hu X, Lin S, Yu D, Qiu S, Zhang X, Mei R. A preliminary study: the anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol Toxicol. 2010;26(6):499–507.
Ishaque S, Shamseer L, Bukutu C, Vohra S. Rhodiola rosea for physical and mental fatigue: a systematic review. BMC Complement Altern Med. 2012;12(1):1–9.
Guo ZY, Gao LP, Li WW. Protective effect of Rhodiola extract against cisplatin-induced damage to TM4 sertoli cells in mice. Natl J Androl. 2013;19(11):1027–33.
Mao JJ, Xie SX, Zee J, Soeller I, Li QS, Rockwell K, et al. Rhodiola rosea versus sertraline for major depressive disorder: a randomized placebo-controlled trial. Phytomed Int J Phytother Phytopharmacol. 2015;22(3):394–9.
Zou H, Liu X, Han T, Hu D, Wang Y, Yuan Y, et al. Salidroside protects against cadmium-induced hepatotoxicity in rats via GJIC and MAPK pathways. PLoS One. 2014;10(6):e0129788. https://doi.org/10.1371/journal.pone.0129788.
Wu YL, Piao DM, Han XH, Nan JX. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol Pharm Bull. 2008;31(8):1523–9.
Zhu Y, Shi YP, Wu D, Ji YJ, Wang X, Chen HL, et al. Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol. 2011;30(10):809–19.
Tan CB, Mei G, Xu WR, Yang XY, Zhu XM, Du GH. Protective effects of salidroside on endothelial cell apoptosis induced by cobalt chloride. Biol Pharm Bull. 2009;32(8):1359–63.
Fang DL, Chen Y, Xu B, Ren K, He ZY, He LL, et al. Development of lipid-shell and polymer core nanoparticles with water-soluble salidroside for anti-cancer therapy. Int J Mol Sci. 2014;15(3):3373–88.
Gavrilov AS, Shchegolev AA, Larionov LP. New prophylactic preparations of Rhodiola rosea. Pharm Chem J. 2003;37(12):641–4.
Yue GC, Chen LH, Guan YM, Zhang MM, Tang S. Micromeritics evaluation of new direct compression excipients. China Pharm. 2014;25(9):833–6.
European Pharmacopoeia, 8th edn. Strasbourg: Council of Europe; 2014.
Bhavsar PH, Bhatt B, Oza C, Trivedi S, Shah S. A review on: SeDeM expert system in formulation development of pharmaceutical forms. J Pharm Sci Bioscientific Res. 2015;5(4):363–9.
Pérez P, Suñé-Negre JM, Miñarro M, Roig M, Fuster R, García-Montoya E, et al. A new expert systems (SeDeM diagram) for control batch powder formulation and preformulation drug products. Eur J Pharm Biopharm. 2006;64(3):351–9.
Aguilardíaz JE, Garcíamontoya E, Pérezlozano P, Suñenegre JM, Miñarro M, Ticó JR. The use of the SeDeM diagram expert system to determine the suitability of diluents–disintegrants for direct compression and their use in formulation of ODT. Eur J Pharm Biopharm. 2009;73(3):414–23.
Raymond CR, Paul JS, Marian EQ. Handbook of pharmaceutical excipients. 6th ed. London: the Pharmaceutical Press; 2009.
European Pharmacopeia, 8th edn. Strasbourg: Dosage forms monographs; 2013.
Suñé-Negre JM, Roig M, Fuster R, Hernández C, Ruhí R, García-Montoya E, et al. New classification of directly compressible (DC) excipients in function of the SeDeM diagarm expert system. Int J Pharm. 2014;470(1–2):15–27.
Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press; 2015.
USP 32/NF 27, The United States Pharmacopeia, 32ª Rev. and The National Formulary, 27th edn. In: USP Monographs: 1217: Tablet Breaking Force, editor. Rockville: United States Pharmacopeial Convention, Inc; 2009.
European Pharmacopeia, 7th edn. Strasbourg: Council of Europe; 2011.
USP 32/NF 27, The United States Pharmacopeia, 32ª rev and The National Formulary, 27th edn. In: USP Monographs: 1216: Tablet Friability, editor. Rockville: United States Pharmacopoeial Convention, Inc; 2009.
United States Pharmacopeia, 32nd Revision. Rockville: Convention, Inc; 2009.
Gregory Thoorens FK, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment-a review. Int J Pharm. 2014;473:64–72.
Rojas J, Ciro Y, Correa L. Functionality of chitin as a direct compression excipient: an acetaminophen comparative study. Carbohydr Polym. 2014;103(1):134–9.
Kato Y, Ohkuma M, Shimada Y, Sunada H. Evaluation of the flowability of surface-modified preparations by the measurement of the inter-particle adhesive force. J Drug Deliv Sci Technol. 2005;15(3):217–21.
Ileleji KE, Zhou B. The angle of repose of bulk corn stover particles. Powder Technol. 2008;187(2):110–8.
Yan D, Zhao LJ, Yi F, Xu DS. Compression characterization of the mixture of direct compression excipients and traditional Chinese medicine (TCM) extract powders. Med Plant. 2013;4(5):9–14.
Singh I, Kumar P. Preformulation studies for direct compression suitability of cefuroxime axetil and paracetamol: a graphical representation using SeDeM diagram. Acta Pol Pharm. 2012;69(1):87.
Acknowledgements
We wish to thank Anhui Shanhe Medicinal Excipients Co., Ltd., China, and Meggle Pharma-Excipients & Technology Co., Ltd., Germany, for providing the necessary materials to carry out this study. This work has emanated from research conducted with the financial support of the National Science & Technology Major Project Key New Drug Creation and Manufacturing Program (2015ZX09303001002007), the Industrial Key Research and Development Project of the Chongqing Science & Technology Commission (cstc2017zdcy-cdyf193), and the Postgraduate Science and Technology Innovation Program of Chongqing University of Science and Technology (YKJCX1820507).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The authors alone are responsible for the content and writing of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, S., Yang, R., Zhang, H. et al. Application of the SeDeM Expert System in Studies for Direct Compression Suitability on Mixture of Rhodiola Extract and an Excipient. AAPS PharmSciTech 20, 105 (2019). https://doi.org/10.1208/s12249-019-1320-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1320-4