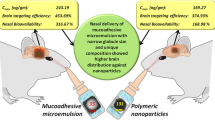
ABSTRACT
To evaluate the possibility of improved drug delivery of quetiapine fumarate (QTP), a nanoemulsion system was developed for intranasal delivery. Effects of different HLBs of Emalex LWIS 10, PEG 400 and Transcutol P, as co-surfactants, were studied on isotropic region of pseudoternary-phase diagrams of nanoemulsion system composed of capmul MCM (CPM) as oil phase, Tween 80 as surfactant and water. Phase behaviour, globule size, transmission electron microscope (TEM) photographs and brain-targeting efficiency of quetiapine nanoemulsion were investigated. In vitro dissolution study of optimised nanoemulsion formulation, with mean diameter 144 ± 0.5 nm, showed more than twofold increase in drug release as compared with pure drug. According to results of in vivo tissue distribution study in Wistar rats, intranasal administration of QTP-loaded nanoemulsion had shorter T max compared with that of intravenous administration. Higher drug transport efficiency (DTE%) and direct nose-to-brain drug transport (DTP%) was achieved by nanoemulsion. The nanoemulsion system may be a promising strategy for brain-targeted delivery of QTP.






Similar content being viewed by others
REFERENCES
Freedman R. Schizophrenia. New Engl J Med. 2003;349(18):1738–49.
Siegfried K, Johannes T, Angela H. Quetiapine: efficacy and tolerability in schizophrenia. Eur Neuropsychopharmacol. 2001;11(4):S405–13.
Estevez-Carrizo FE, Parrillo Ercoli MC, Estevez-Parrillo FT. single-dose relative bioavailability of a new quetiapine fumarate extended-release formulation: a postprandial, randomized, open-label, two-period crossover study in healthy uruguayan volunteers. Clin Ther. 2011;33(6):738–45.
Hsiao CC, Chen KP, Tsai CJ, Wang LJ, Chen CK, Lin SK. Rapid initiation of quetiapine well tolerated as compared with the conventional initiation regimen in patients with schizophrenia or schizoaffective disorders. Kaohsiung J Med Sci. 2011;27(11):508–13.
Sharma T. Quetiapine—efficacy in different domains. Eur Neuropsychopharmacol. 2001;11(4):S385–90.
Pae CU, Kim TS, Kim JJ, Lee SJ, Lee CU, Lee C, et al. Long-term treatment of adjunctive quetiapine for bipolar mania. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):763–6.
Wetzel H, Szegedi A, Hain C, Wiesner J. Seroquel (ICI 204 636), a putative atypical antipsychotic, in schizophrenia with positive symptomatology: results of an open clinical trial and changes of neuroendocrinological and EEG parameters. Psychopharmacology (Berl). 1995;119(2):231–8.
Goren JL, Levin GM. Quetiapine, an atypical antipsychotic. Pharmacotherapy. 1998;18(6):1183–94.
Green B. Focus on quetiapine. Curr Med Res Opin. 1999;15(3):145–51.
Thyrum PT, Wong YWJ, Yeh C. Single dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairement. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(4):521–33.
Dhuria SV, Hanson LR, Frey W. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Wu H, Hu K, Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Deliv. 2008;5(10):1159–68.
Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target. 2010;18(3):223–34.
Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Brain targeting studies on buspirone hydrochloride after intranasal administration of mucoadhesive formulation in rats. J Pharm Pharmacol. 2009;61(5):669–75.
Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci. 2006;95(3):570–80.
Vyas TK, Babbar AK, Sharma RK, Misra A. Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target. 2005;13(5):317–24.
Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2001;53(1):3–21.
Devarajan V, Ravichandran V. Nanoemulsions: as modified drug delivery tool. Int J Comprehensive Pharm. 2011;2(4):1–6.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Kelmann RG, Kuminek G, Teixeira HF, Koester LS. Carbamazepine parenteral nanoemulsions prepared by spontaneous emulsification process. Int J Pharm. 2007;342(1-2):231–9.
Kaltsa O, Michon C, Yanniotis S, Mandala I. Ultrasonic energy input influence on the production of sub-micron o/w emulsions containing whey protein and common stabilizers. Ultrason Sonochem. 2013;20:881–9.
Yuan Y, Yanxiang G, Jian Z, Like M. Characterization and stability evaluation of b-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int. 2008;41(1):61–8.
Piaoa HM, Balakrishnana P, Choa HJ, Kimb H, Kimb YS, Chunga SJ, et al. Preparation and evaluation of fexofenadine microemulsions for intranasal Delivery. Int J Pharm. 2010;395(1–2):309–16.
Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2013; Early Online: 1–7.
Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–79.
Gadiko C, Pamu P, Kilari EK. Effect of amiodarone on the pharmacodynamics of gliclazide in animal models. Int J Pharm Pharm Sci. 2013;5(4):290–3.
Patil-Gadhe A, Pokharkar V. Montelukast loaded nanostructured lipid carriers: part I oral bioavailability improvement. Eur J Pharm Biopharm. 2014;88(1):160–8.
Flieger J, Pizon M, Plech T, Luszczki JJ. Analysis of new potential anticonvulsant compounds in mice brain tissue by SPE/HPLC/DAD. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:26–33.
Xu S, Zheng S, Shen X, Yao Z, Pivnichny J, Tong X. Automated sample preparation and purification of homogenized brain tissues. J Pharm Biomed Anal. 2007;44(2):581–5.
Davis PC, Wong J, Gefvert O. Analysis and pharmacokinetics of quetiapine and two metabolites in human plasma using reversed-phase HPLC with ultraviolet and electrochemical detection. J Pharm Biomed Anal. 1999;20:271–82.
Qizhi Z, Xinguo J, Wenming J, Wei L, Lina S, Zhenqi S. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275(1-2):85–96.
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target. 2008;16(10):806–14.
Hyun JC, Wan SK, Ubonvan T, Insoo Y, Chung WC, Hyun TM, et al. Development of udenafil-loaded microemulsions for intranasal delivery: in-vitro and in-vivo evaluations. Int J Pharm. 2012;423(2):153–60.
Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs I. prescription design. J Contrl Release. 2002;81(1–2):65–74.
Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, et al. Microemulsion formulation for enhanced absorption of poorly soluble drugs II. In-vivo study. J Control Release. 2002;81(1–2):75–82.
Li L, Nandi I, Kim KH. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm. 2002;237(1–2):77–85.
Sheikh S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227–43.
Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocolloid. 2011;25(5):1000–8.
Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–65.
Varshosaz J, Eskandari S, Tabakhian M. Production and optimization of valproic acid nanostructured lipid carriers by the Taguchi design. Pharm Dev Technol. 2010;15(1):89–96.
Kiruba F, Lalan M, Babbar AK, Kaul A, Mishra AK, Misra A. Intranasal Clobazam delivery in the treatment of status epilepticus. J Pharm Sci. 2010;100(2):692–703.
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm. 2008;358(1–2):285–91.
ACKNOWLEDGMENTS
The authors are thankful to AICTE for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Interest
The authors report no declarations of interest.
Rights and permissions
About this article
Cite this article
Boche, M., Pokharkar, V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 18, 686–696 (2017). https://doi.org/10.1208/s12249-016-0552-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0552-9