Abstract
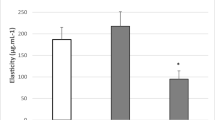
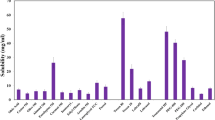
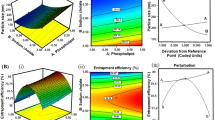
Curcumin has diverse biological activities including antioxidant and anti-inflammatory activity. However, its clinical use for topical application is limited due to its poor aqueous solubility and thus, minimal cutaneous bioavailability. Elastic vesicles (EVs) of curcumin were prepared to improve its cutaneous bioavailability and to use it for topical anti-inflammatory effect. Ex vivo skin permeation and retention studies were performed to check if incorporation of curcumin into EVs could improve its permeation into and retention in the skin. Evaluation of acute and chronic anti-inflammatory effect was done using xylene-induced acute ear edema in mice and cotton pellet-induced chronic inflammation in rats, respectively. A significant improvement in flux (nine times) across murine skin was observed when aqueous dispersion of curcumin (flux − 0.46 ± 0.02 μg/h/cm2) was compared with curcumin-loaded EVs (flux − 4.14 ± 0.04 μg/h/cm2 ). Incorporation of these curcumin-loaded EVs into a hydrophilic ointment base resulted in higher skin retention (51.66%) in contrast to free curcumin ointment (1.64%) and a marketed formulation (VICCO® turmeric skin cream). The developed ointment showed an effect similar (p < 0.05) to the marketed diclofenac sodium ointment (Omni-gel®) in suppression of acute inflammation in mouse; a significant inhibition (28.8% versus 3.91% for free curcumin) of cotton pellet-induced chronic inflammation was also observed. Thus, curcumin-loaded EVs incorporated in hydrophilic ointment is a promising topical anti-inflammatory formulation.







Similar content being viewed by others
REFERENCES
Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223(2):181–90.
Hsuuw YD, Chang CK, Chan WH, Yu JS. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J Cell Physiol. 2005;205(3):379–86.
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98.
Sun J, Zhao Y, Hu J. Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One. 2013;8(6):e67078.
Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25.
Deng S-L, Chen W-F, Zhou B, Yang L, Liu Z-L. Protective effects of curcumin and its analogues against free radical-induced oxidative haemolysis of human red blood cells. Food Chem. 2006;98(1):112–9.
Osawa T, Kato Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann N Y Acad Sci. 2005;1043:440–51.
Strasser EM, Wessner B, Manhart N, Roth E. The relationship between the anti-inflammatory effects of curcumin and cellular glutathione content in myelomonocytic cells. Biochem Pharmacol. 2005;70(4):552–9.
Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2010;55(3):495–503.
Kaur IP, Kapila M, Agrawal R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res Rev. 2007;6(4):271–88.
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila). 2011;4(8):1158–71.
Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49(11):2906–13.
Bhushan S, Kakkar V, Pal HC, Guru SK, Kumar A, Mondhe DM, et al. Enhanced anticancer potential of encapsulated solid lipid nanoparticles of TPD: a novel triterpenediol from Boswellia serrata. Mol Pharm. 2012;10(1):225–35.
Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17(5):5972–87.
Zhao YZ, Lu CT, Zhang Y, Xiao J, Zhao YP, Tian JL, et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int J Pharm. 2013;454(1):302–9.
Gallarate M, Chirio D, Trotta M, Eugenia Carlotti M. Deformable liposomes as topical formulations containing alpha-tocopherol. J Dispers Sci Technol. 2006;27(5):703–13.
Castangia I, Nacher A, Caddeo C, Valenti D, Fadda AM, Diez-Sales O, et al. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2013;10(3):1292–300.
Chaudhary H, Kohli K, Kumar V. Nano-transfersomes as a novel carrier for transdermal delivery. Int J Pharm. 2013;454(1):367–80.
Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35(10):3365–83.
Agrawal R, Kaur IP. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res. 2010;13(4):397–410.
Cevc G, Blume G, Schätzlein A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo. J Control Release. 1997;45(3):211–26.
El Maghraby GM, Williams AC, Barry BW. Skin hydration and possible shunt route penetration in controlled estradiol delivery from ultradeformable and standard liposomes. J Pharm Pharmacol. 2001;53(10):1311–22.
El Maghraby GMM, Williams AC, Barry BW. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int J Pharm. 2004;276:143–61.
Essa EA, Bonner MC, Barry BW. Iontophoretic estradiol skin delivery and tritium exchange in ultradeformable liposomes. Int J Pharm. 2002;240(1–2):55–66.
Ma Y, Li Y, Li X, Wu Y. Anti-inflammatory effects of 4-methylcyclopentadecanone on edema models in mice. Int J Mol Sci. 2013;14(12):23980–92.
Niu X, Li Y, Li W, Hu H, Yao H, Li H, et al. The anti-inflammatory effects of Caragana tangutica ethyl acetate extract. J Ethnopharmacol. 2014;152(1):99–105.
Swingle KF, Shideman FE. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther. 1972;183(1):226–34.
Fabri RL, Garcia RA, Florencio JR, de Castro Campos Pinto N, de Oliveira LG, Aguiar JA, et al. Anti-inflammatory and antioxidative effects of the methanolic extract of the aerial parts of Mitracarpus frigidus in established animal models. J Pharm Pharmacol. 2013;66(5):722–33.
Ntimenou V, Fahr A, Antimisiaris SG. Elastic vesicles for transdermal drug delivery of hydrophilic drugs: a comparison of important physicochemical characteristics of different vesicle types. J Biomed Nanotechnol. 2012;8(4):613–23.
Uchino T, Lefeber F, Gooris G, Bouwstra J. Physicochemical characterization of drug-loaded rigid and elastic vesicles. Int J Pharm. 2011;412(1–2):142–7.
van den Bergh BA, Wertz PW, Junginger HE, Bouwstra JA. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int J Pharm. 2001;217(1–2):13–24.
Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm. 2010;76:475–85.
Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies. VI: preparation of the barrier layer. J Pharm Sci. 1986;75(5):487–91.
Catz P, Friend DR. Transdermal delivery of levonorgestrel. VIII. Effect of enhancers on rat skin, hairless mouse skin, hairless guinea pig skin, and human skin. Int J Pharm. 1990;58(2):93–102.
Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies III: hydrophobic compounds. J Pharm Sci. 1984;73(9):1255–8.
Cevc G, Gebauer D, Stieber J, Schatzlein A, Blume G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta. 1998;1368(2):201–15.
Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29(9):1013–26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, R., Sandhu, S.K., Sharma, I. et al. Development and Evaluation of Curcumin-loaded Elastic Vesicles as an Effective Topical Anti-inflammatory Formulation. AAPS PharmSciTech 16, 364–374 (2015). https://doi.org/10.1208/s12249-014-0232-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0232-6