Abstract
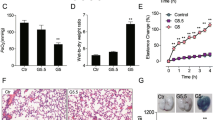
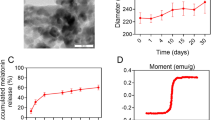
Formoterol is a long-acting β2 agonist (LABA). Agonism of the β2-adrenergic receptor by formoterol is known to stimulate mitochondrial biogenesis (MB) in renal proximal tubules and recover kidney function. However, formoterol has a number of cardiovascular side effects that limits its usage. The goal of this study was to design and develop an intravenous biodegradable and biocompatible polymeric nanoparticle delivery system that targets formoterol to the kidney. Poly(ethylene glycol) methyl ether-block-poly(lactide-co-glycolide) nanoparticles containing encapsulated formoterol were synthesized by a modified single-emulsion solvent evaporation technique resulting in nanoparticles with a median hydrodynamic diameter of 442 + 17 nm. Using primary cell cultures of rabbit renal proximal tubular cells (RPTCs), free formoterol, encapsulated formoterol polymeric nanoparticles, and drug-free polymeric nanoparticles were biocompatible and not cytotoxic over a wide concentration range. In healthy male mice, polymeric nanoparticles were shown to localize in tubules of the renal cortex and improved the renal localization of encapsulated formoterol compared to the free formoterol. At a lower total formoterol dose, the nanoparticle localization resulted in increased expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), the master regulator of MB, and increased electron transport chain proteins, markers of MB. This was confirmed by direct visual quantification of mitochondria and occurred with both free formoterol and the encapsulated formoterol polymeric nanoparticles. At the same time, localization of nanoparticles to the kidneys resulted in reduced induction of MB markers in the heart. These new nanoparticles effectively target formoterol to the kidney and successfully produce MB in the kidney.







Similar content being viewed by others
References
Feldkamp T, Kribben A, Weinberg JM. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Ren Physiol. 2005;288(6):F1092–102.
Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–58.
Cleveland KH, Brosius FC, Schnellmann RG. Regulation of mitochondrial dynamics and energetics in the diabetic renal proximal tubule by the β2-adrenergic receptor agonist formoterol. Am J Physiol Ren Physiol. 2020;319(5):F773–F9.
Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–66.
Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13(10):629–46.
Rasbach KA, Schnellmann RG. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem. 2007;282(4):2355–62.
Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97(6):2826–31.
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–73.
Golestaneh L, Melamed ML, Hostetter TH. Uremic memory: the role of acute kidney injury in long-term outcomes. Kidney Int. 2009;76(8):813–4.
Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355(3):734–9.
Bhargava P, Janda J, Schnellmann RG. Elucidation of cGMP-dependent induction of mitochondrial biogenesis through PKG and p38 MAPK in the kidney. Am J Physiol Ren Physiol. 2020;318(2):F322–F8.
Dupre TV, Jenkins DP, Muise-Helmericks RC, Schnellmann RG. The 5-hydroxytryptamine receptor 1F stimulates mitochondrial biogenesis and angiogenesis in endothelial cells. Biochem Pharmacol. 2019;169:113644.
Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–38.
Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333(2):593–601.
Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, et al. The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther. 2012;342(1):106–18.
Cameron RB, Peterson YK, Beeson CC, Schnellmann RG. Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci Rep. 2017;7(1):10578.
Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014;25(6):1157–62.
Cameron RB, Gibbs WS, Miller SR, Dupre TV, Megyesi J, Beeson CC, et al. Proximal Tubule. J Pharmacol Exp Ther. 2019;369(1):173–80.
Arif E, Solanki AK, Srivastava P, Rahman B, Fitzgibbon WR, Deng P, et al. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 2019;96(3):656–73.
Boivin V, Jahns R, Gambaryan S, Ness W, Boege F, Lohse MJ. Immunofluorescent imaging of beta 1- and beta 2-adrenergic receptors in rat kidney. Kidney Int. 2001;59(2):515–31.
Arif E, Nihalani D. Beta2-adrenergic receptor in kidney biology: A current prospective. Nephrology (Carlton). 2019;24(5):497–503.
Levine MA, Leenen FH. Role of beta 1-receptors and vagal tone in cardiac inotropic and chronotropic responses to a beta 2-agonist in humans. Circulation. 1989;79(1):107–15.
Brodde OE. Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43(2):203–42.
Vyas FS, Nelson CP, Freeman F, Boocock DJ, Hargreaves AJ, Dickenson JM. β 2-adrenoceptor-induced modulation of transglutaminase 2 transamidase activity in cardiomyoblasts. Eur J Pharmacol. 2017;813:105–21.
Molenaar P, Chen L, Parsonage WA. Cardiac implications for the use of beta2-adrenoceptor agonists for the management of muscle wasting. Br J Pharmacol. 2006;147(6):583–6.
Yin Q, Yang C, Wu J, Lu H, Zheng X, Zhang Y, et al. Downregulation of β-adrenoceptors in isoproterenol-induced cardiac remodeling through HuR. PLoS One. 2016;11(4):e0152005.
Koziczak-Holbro M, Rigel DF, Dumotier B, Sykes DA, Tsao J, Nguyen NH, et al. Pharmacological characterization of a novel 5-hydroxybenzothiazolone-derived. J Pharmacol Exp Ther. 2019;369(2):188–99.
Mansour HM, Sohn M, Al-Ghananeem A, P.P. D. Materials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release drug delivery aspects. . International Journal of Molecular Sciences. 2010;11(special issue-material sciences and nanotechnology section - biodegradability of materials.):3298-32
Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377–97.
Rhee YS, Park CW, DeLuca PP, Mansour HM. Sustained-release injectable drug delivery systems. . Pharmaceutical Technology: Special Issue-Drug Delivery. 2010( November): 6-13
Hasanpour A, Esmaeili F, Hosseini H, Amani A. Use of mPEG-PLGA nanoparticles to improve bioactivity and hemocompatibility of streptokinase: in-vitro and in-vivo studies. Mater Sci Eng C Mater Biol Appl. 2021;118:111427.
Muralidharan P, Mallory E, Malapit M, Hayes D Jr, Mansour HM. Inhalable PEGylated phospholipid nanocarriers and PEGylated therapeutics for respiratory delivery as aerosolized colloidal dispersions and dry powder inhalers. Pharmaceutics. 2014;6(2):333–53.
Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8(6):2101–41.
Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23.
Sasaki H, Kamimura H, Shiobara Y, Esumi Y, Takaichi M, Yokoshima T. Disposition and metabolism of formoterol fumarate, a new bronchodilator, in rats and dogs. Xenobiotica. 1982;12(12):803–12.
Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. Arterial uptake of biodegradable nanoparticles: effect of surface modifications. J Pharm Sci. 1998;87(10):1229–34.
Kumar A, Mansour HM, Friedman A, Blough E. Nanomedicine in Drug Delivery. 1st ed: CRC Press; 2017
Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98.
Song CX, Labhasetwar V, Murphy H, Qu X, Humphry WR, Shebuski RJ, et al. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J Control Release. 1997;43:197–212.
Westedt U, Kalinowski M, Wittmar M, Merdan T, Unger F, Fuchs J, et al. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Control Release. 2007;119(1):41–51.
He J, Chen H, Zhou W, Chen M, Yao Y, Zhang Z, et al. Kidney targeted delivery of asiatic acid using a FITC labeled renal tubular-targeting peptide modified PLGA-PEG system. Int J Pharm. 2020;584:119455.
Yu H, Lin T, Chen W, Cao W, Zhang C, Wang T, et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials. 2019;219:119368.
Mansour HM, Park CW. Book Chapter: Therapeutic and clinical aspects of nanomedicines and nanopharmaceutical products. In: Brenner S, editor. The Nanomedicine Handbook for Clinicians 1. London. United Kingdom: CRC Press, Inc.; 2011.
Williams RM, Jaimes EA, Heller DA. Nanomedicines for kidney diseases. Kidney Int. 2016;90(4):740–5.
Nair AV, Keliher EJ, Core AB, Brown D, Weissleder R. Characterizing the interactions of organic nanoparticles with renal epithelial cells in vivo. ACS Nano. 2015;9(4):3641–53.
Williams RM, Shah J, Ng BD, Minton DR, Gudas LJ, Park CY, et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015;15(4):2358–64.
Williams RM, Shah J, Tian HS, Chen X, Geissmann F, Jaimes EA, et al. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension. 2018;71(1):87–94.
Han SJ, Williams RM, D'Agati V, Jaimes EA, Heller DA, Lee HT. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020;98(1):76–87.
Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27(21):4025–33.
Duan JH, Mansour HM, Zhang YD, Deng XM, Zhao JF. Reversion of multi-drug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butylcyanoacrylate) nanoparticles. Int J Pharm. 2012:1–9.
Mascher DG, Zech K, Nave R, Kubesch KM, Mascher HJ. Ultra-sensitive determination of Formoterol in human serum by high performance liquid chromatography and electrospray tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2006;830(1):25–34.
Alabsi W, Al-Obeidi FA, Polt R, Mansour HM. Organic solution advanced spray-dried microparticulate/nanoparticulate dry powders of lactomorphin for respiratory delivery: physicochemical characterization, in vitro aerosol dispersion, and cellular studies. Pharmaceutics. 2021;13:26.
Park CW, Li X, Vogt FG, Hayes D Jr, Zwischenberger JB, Park ES, et al. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int J Pharm. 2013;455(1-2):374–92.
Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Phys. 1995;268(4 Pt 1):C1053–61.
Acosta MF, Abrahamson MD, Encinas-Basurto D, Fineman JR, Black SM, Mansour HM. Inhalable nanoparticles/microparticles of an AMPK and Nrf2 activator for targeted pulmonary drug delivery as dry powder inhalers. AAPS J. 2020;23(1):2.
Isoe J, Collins J, Badgandi H, Day WA, Miesfeld RL. Defects in coatomer protein I (COPI) transport cause blood feeding-induced mortality in yellow fever mosquitoes. Proc Natl Acad Sci U S A. 2011;108(24):E211–7.
Zuckerman JE, Davis ME. Targeting therapeutics to the glomerulus with nanoparticles. Adv Chronic Kidney Dis. 2013;20(6):500–7.
Cartiera MS, Johnson KM, Rajendran V, Caplan MJ, Saltzman WM. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30(14):2790–8.
Bernstein D, Fajardo G, Zhao M. The role of β-adrenergic receptors in heart failure: differential regulation of cardiotoxicity and cardioprotection. Prog Pediatr Cardiol. 2011;31(1):35–8.
Brouri F, Findji L, Mediani O, Mougenot N, Hanoun N, Le Naour G, et al. Toxic cardiac effects of catecholamines: role of beta-adrenoceptor downregulation. Eur J Pharmacol. 2002;456(1-3):69–75.
de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369(26):2492–503.
Acknowledgements
The authors gratefully acknowledge University of Arizona funding. The authors are grateful to Yelena Feinstein and Krishna Parsawar for assistance with the mass spectrometry as well as the laboratory of Dr. Jianqin Lu for use of their rotary evaporator.
Funding
This work was funded in part by support from the University of Arizona College of Pharmacy as well as the Southwest Environmental Health Sciences Center (P30 ES006694).
All SEM images and data were collected in the W.M. Keck Center for Nano-Scale Imaging in the Department of Chemistry and Biochemistry at the University of Arizona with funding from the W.M. Keck Foundation Grant.
All TEM images and data were collected in The University of Arizona, Imaging Cores - Life Sciences North with funding from the NIH (S10 OD011981) and assistance from Dr. William A. Day.
Mass Spectra were acquired by the Arizona Analytical and Biological Mass Spectrometry Core Facility supported by NIEHS grant ES06694 to the Southwest Environmental Health Sciences Center, NIH/NCI grant CA023074 to the Arizona Cancer Center, and by the BIO5 Institute of the University of Arizona.
Author information
Authors and Affiliations
Contributions
Ernest L. Vallorz: Conceptualization, methodology, data acquisition, interpretation, analysis, writing — original draft. Karen Blohm-Mangone: methodology, data acquisition. Rick G. Schnellmann: conceptualization; methodology; interpretation; analysis; writing — review and editing; resources; funding acquisition; resources; supervision; project administration. Heidi M. Mansour: conceptualization; methodology; interpretation; analysis; writing — review and editing; resources; supervision; project administration
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vallorz, E.L., Blohm-Mangone, K., Schnellmann, R.G. et al. Formoterol PLGA-PEG Nanoparticles Induce Mitochondrial Biogenesis in Renal Proximal Tubules. AAPS J 23, 88 (2021). https://doi.org/10.1208/s12248-021-00619-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00619-4