Abstract
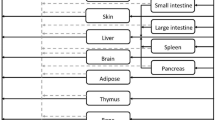
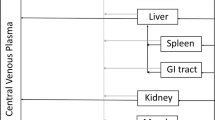
Quantitative modeling of the subcutaneous absorption processes of protein therapeutics is challenging. Here we have proposed a “two-pore” PBPK model that is able to simultaneously characterize plasma PK of different-size protein therapeutics in mice. The skin compartment is evolved to mechanistically account for the absorption pathways through lymph and blood capillaries, as well as local degradation at the SC injection site. The model is developed using in-house plasma PK data generated following subcutaneous administration of 6 different-size protein therapeutics (13–150 kDa) in mice. The model was able to capture plasma PK of all molecules following intravenous and subcutaneous administration relatively well. From the observed plasma PK profiles, as well as from the model simulation result, several important PK descriptors were found to be dependent on protein size for FcRn nonbinding molecules. A positive correlation was found between Tmax and protein size. A “U” shape relationship was found between Cmax and protein size. Negative correlations were observed between bioavailability (F) and local degradation rate (kdeg,SC), and F and protein size. Pathway analysis of the model was conducted for the subcutaneous absorption process, and continuous relationships were established between the percentage of absorption through lymphatic and vascular pathways and protein size. This PBPK model could serve as a platform for the development of different-size protein therapeutics and will be scaled up to humans for translational studies in the future.




Similar content being viewed by others
References
Viola M, Sequeira J, Seiça R, Veiga F, Serra J, Santos AC, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–14.
Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos. 2014;42(11):1881–9.
Zhao L, Ji P, Li Z, Roy P, Sahajwalla CG. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol. 2013;53(3):314–25.
Gill KL, Gardner I, Li L, Jamei M. A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J. 2016;18(1):156–70.
Hu S, D’Argenio DZ. Predicting monoclonal antibody pharmacokinetics following subcutaneous administration via whole-body physiologically-based modeling. J Pharmacokinet Pharmacodyn. 2020;47:385–409.
Li Z, Shah DK. Two-pore physiologically based pharmacokinetic model with de novo derived parameters for predicting plasma PK of different size protein therapeutics. J Pharmacokinet Pharmacodyn. 2019;46(3):305–18.
Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994;74(1):163–219.
Sepp A, Berges A, Sanderson A, Meno-Tetang G. Development of a physiologically based pharmacokinetic model for a domain antibody in mice using the two-pore theory. J Pharmacokinet Pharmacodyn. 2015;42(2):97–109.
Li Z, Li Y, Chang H-P, Chang H-Y, Guo L, Shah DK. Effect of size on solid tumor disposition of protein therapeutics. Drug Metab Dispos. 2019;47(10):1136–45.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86.
Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–87.
Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54(6):1517–28.
Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59.
Boswell CA, Mundo EE, Ulufatu S, Bumbaca D, Cahaya HS, Majidy N, et al. Comparative physiology of mice and rats: radiometric measurement of vascular parameters in rodent tissues. Mol Pharm. 2014;11(5):1591–8.
Yoshioka E, Kato K, Shindo H, Mitsuoka C, Kitajima S-I, Ogata H, et al. Pharmacokinetic study of darbepoetin alfa: absorption, distribution, and excretion after a single intravenous and subcutaneous administration to rats. Xenobiotica. 2007;37(1):74–90.
Sepp A, Meno-Tetang G, Weber A, Sanderson A, Schon O, Berges A. Computer-assembled cross-species/cross-modalities two-pore physiologically based pharmacokinetic model for biologics in mice and rats. J Pharmacokinet Pharmacodyn. 2019;46(4):339–59.
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35(9):1672–8.
Wu F, Bhansali SG, Law WC, Bergey EJ, Prasad PN, Morris ME. Fluorescence imaging of the lymph node uptake of proteins in mice after subcutaneous injection: molecular weight dependence. Pharm Res. 2012;29(7):1843–53.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Kagan L, Mager DE. Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab Dispos. 2013;41(1):248–55.
Davis CB, Bugelski PJ. Subcutaneous bioavailability of a PRIMATIZEDTM IgG1 Anti-Human CD4 monoclonal antibody is dose dependent in transgenic mice bearing human CD4. Drug Deliv. 1998;5(2):95–100.
Deng R, Meng YG, Hoyte K, Lutman J, Lu Y, Iyer S, et al. Subcutaneous bioavailability of therapeutic antibodies as a function of FcRn binding affinity in mice. In MAbs, Taylor & Francis. 2012;4(1):101–09.
Zheng Y, Tesar DB, Benincosa L, Birnböck H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. In MAbs, Taylor & Francis. 2012;4(2):243–55.
Fathallah AM, Turner MR, Mager DE, Balu-Iyer SV. Effects of hypertonic buffer composition on lymph node uptake and bioavailability of rituximab, after subcutaneous administration. Biopharm Drug Dispos. 2015;36(2):115–25.
Richter WF, Christianson GJ, Frances N, Grimm HP, Proetzel G, Roopenian DC. Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice. In MAbs, Taylor & Francis. 2018;10(5):803–13.
Datta-Mannan A, Witcher DR, Lu J, Wroblewski VJ. Influence of improved FcRn binding on the subcutaneous bioavailabilityof monoclonal antibodies in cynomolgus monkeys. In MAbs, Taylor & Francis. 2012;4(2):267–73.
Takeyama M, Ishida T, Kokubu N, Komada F, Iwakawa S, Okumura K, et al. Enhanced bioavailability of subcutaneously injected insulin by pretreatment with ointment containing protease inhibitors. Pharm Res. 1991;8(1):60–4.
Wang W, Chen N, Shen X, Cunningham P, Fauty S, Michel K, et al. Lymphatic transport and catabolism of therapeutic proteins after subcutaneous administration to rats and dogs. Drug Metab Dispos. 2012;40(5):952–62.
Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9.
Acknowledgements
This work was supported by the National Institute of General Medical Sciences grant [GM114179]. D.K.S is also supported by and National Institute of Allergy and Infectious Diseases grant [AI138195] and National Cancer Institute grant [R01CA246785].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Yu, X., Li, Y. et al. A Two-Pore Physiologically Based Pharmacokinetic Model to Predict Subcutaneously Administered Different-Size Antibody/Antibody Fragments. AAPS J 23, 62 (2021). https://doi.org/10.1208/s12248-021-00588-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00588-8