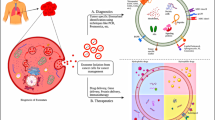
Abstract
Exosomes are extracellular microvesicles with a particle size of 30–100 nm and carry a cargo of proteins, lipids, RNA, and DNA. Their properties of shuttling in-and-out of the cells suggest that these particles can be exploited as a nano drug carrier. In this manuscript, we show that curcumin can be delivered effectively using milk-derived exosomes. Curcumin when mixed with exosomes in the presence of 10% ethanol:acetonitrile (1:1) provided a drug load of 18–24%, and the formulation stored at − 80°C was stable for 6 months as determined by particle size analysis, drug load, and antiproliferative activity. The uptake of exosomes by cancer cells involved caveolae/clathrin-mediated endocytosis. Oral administration of exosomal curcumin (ExoCUR) in Sprague-Dawley rats demonstrated 3–5 times higher levels in various organs versus free agent. ExoCUR showed enhanced antiproliferative activity against multiple cancer cell lines including, breast, lung, and cervical cancer compared with the free curcumin. ExoCUR showed significantly higher anti-inflammatory activity measured as NF-κB activation in human lung and breast cancer cells. To determine in vivo antitumor activity, nude mice bearing the cervical CaSki tumor xenograft were treated with ExoCUR by oral gavage, curcumin diet, exosomes alone, and PBS as controls. While curcumin via dietary route failed to elicit any effect, exosomes had a modest (25–30%) tumor growth inhibition. However, ExoCUR showed significant inhibition (61%; p < 0.01) of the cervical tumor xenograft. No gross or systemic toxicity was observed in the rats administered with the exosomes or ExoCUR. These results suggest that exosomes can be developed as potential nano carriers for delivering curcumin which otherwise has encountered significant tissue bioavailability issues in the past.








Similar content being viewed by others
References
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–64. https://doi.org/10.1016/j.canlet.2008.03.025.
Bansal SS, Kausar H, Vadhanam MV, Ravoori S, Pan J, Rai SN, et al. Curcumin implants, not curcumin diet, inhibit estrogen-induced mammary carcinogenesis in ACI rats. Cancer Prev Res. 2014;7(4):456–65. https://doi.org/10.1158/1940-6207.CAPR-13-0248.
Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2012;69(3):679–89. https://doi.org/10.1007/s00280-011-1749-y.
Wang X, Wang Y, Chen ZG, Shin DM. Advances of cancer therapy by nanotechnology. Cancer Res Treat. 2009;41(1):1–11. https://doi.org/10.4143/crt.2009.41.1.1.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. https://doi.org/10.1038/nrd1632.
Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. https://doi.org/10.1002/smll.200700595.
Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22. https://doi.org/10.1093/toxsci/kfj130.
Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552–8. https://doi.org/10.1038/sj.bjp.0707130.
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. https://doi.org/10.1055/s-2006-957450.
Grill AE, Koniar B, Panyam J. Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin. Drug Deliv Transl Res. 2014;4(4):344–52. https://doi.org/10.1007/s13346-014-0199-6.
Aqil F, Jeyabalan J, Kausar H, Bansal SS, Sharma RJ, Singh IP, et al. Multi-layer polymeric implants for sustained release of chemopreventives. Cancer Lett. 2012;326(1):33–40. https://doi.org/10.1016/j.canlet.2012.07.017.
Gupta RC, Bansal SS, Aqil F, Jeyabalan J, Cao P, Kausar H, et al. Controlled-release systemic delivery—a new concept in cancer chemoprevention. Carcinogenesis. 2012;33(8):1608–15. https://doi.org/10.1093/carcin/bgs209.
Bansal SS, Kausar H, Aqil F, Jeyabalan J, Vadhanam MV, Gupta RC, et al. Curcumin implants for continuous systemic delivery: safety and biocompatibility. Drug Deliv Transl Res. 2011;1(4):332–41. https://doi.org/10.1007/s13346-011-0028-0.
Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J Drug Target. 2014;22(7):576–83. https://doi.org/10.3109/1061186X.2014.934688.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96. https://doi.org/10.1016/j.apsb.2016.02.001.
Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–41. https://doi.org/10.2147/IJN.S29661ijn-7-1525.
Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33(10):737–41. https://doi.org/10.1002/bies.201100076.
Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, et al. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol. 2016;101(1):12–21. https://doi.org/10.1016/j.yexmp.2016.05.013.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. https://doi.org/10.1016/j.canlet.2015.10.020.
Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, et al. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3:2534. https://doi.org/10.1038/srep02534.
Kausar H, Munagala R, Bansal SS, Aqil F, Vadhanam MV, Gupta RC. Cucurbitacin B potently suppresses non-small-cell lung cancer growth: identification of intracellular thiols as critical targets. Cancer Lett. 2013;332(1):35–45. https://doi.org/10.1016/j.canlet.2013.01.008.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer. Cancer Lett. 2015;356(2 Pt B):536–46. https://doi.org/10.1016/j.canlet.2014.09.037.
Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191(7):1395–411. https://doi.org/10.1083/jcb.201006098.
Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053-1064; https://doi.org/10.1016/j.biotechadv.2014.04.004.
Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–78.
Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396(2):528–33. https://doi.org/10.1016/j.bbrc.2010.04.135S0006-291X(10)00830-2.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. https://doi.org/10.1021/mp700113r.
Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomark Prev. 2002;11(1):105–11.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–9. https://doi.org/10.1158/1078-0432.CCR-08-0024.
Mulik R, Mahadik K, Paradkar A. Development of curcuminoids loaded poly(butyl) cyanoacrylate nanoparticles: physicochemical characterization and stability study. Eur J Pharm Sci. 2009;37(3–4):395–404. https://doi.org/10.1016/j.ejps.2009.03.009.
Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3–4):223–30. https://doi.org/10.1016/j.ejps.2009.02.019.
Yadav VR, Suresh S, Devi K, Yadav S. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J Pharm Pharmacol. 2009;61(3):311–21. https://doi.org/10.1211/jpp/61.03.0005.
Chen C, Johnston TD, Jeon H, Gedaly R, McHugh PP, Burke TG, et al. An in vitro study of liposomal curcumin: stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int J Pharm. 2009;366(1–2):133–9. https://doi.org/10.1016/j.ijpharm.2008.09.009.
Ranjan AP, Mukerjee A, Helson L, Gupta R, Vishwanatha JK. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: inhibition of tumor growth and angiogenesis. Anticancer Res. 2013;33(9):3603–9.
Nayak AP, Mills T, Norton I. Lipid based nanosystems for curcumin: past, present and future. Curr Pharm Des. 2016;22(27):4247–56.
Wang T, Ma X, Lei Y, Luo Y. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcumin. Colloids Surf B Biointerfaces. 2016;148:1–11. https://doi.org/10.1016/j.colsurfb.2016.08.047.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–63. https://doi.org/10.1016/j.ijpharm.2006.09.025.
Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2011;4(8):1158–71. https://doi.org/10.1158/1940-6207.CAPR-10-0006.
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66. https://doi.org/10.1182/blood-2004-03-0824.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–22. https://doi.org/10.1074/jbc.M109.041152.
Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96. https://doi.org/10.1002/jcb.22733.
Tian T, Zhu YL, FH H, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–95. https://doi.org/10.1002/jcp.24304.
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13(5):1627-1636;https://doi.org/10.1016/j.nano.2017.03.001.
Vashisht M, Rani P, Onteru SK, Singh D. Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol. 2017; https://doi.org/10.1007/s12010-017-2478-4.
Liao Y, Du X, Li J, Lonnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017; https://doi.org/10.1002/mnfr.201700082.
Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–24. https://doi.org/10.1093/nar/gkp857.
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–73. https://doi.org/10.1002/mnfr.201300729.
Lei T, Srinivasan S, Tang Y, Manchanda R, Nagesetti A, Fernandez-Fernandez A, et al. Comparing cellular uptake and cytotoxicity of targeted drug carriers in cancer cell lines with different drug resistance mechanisms. Nanomedicine. 2011;7(3):324–32. https://doi.org/10.1016/j.nano.2010.11.004.
Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–9. https://doi.org/10.1158/0008-5472.CAN-14-3095.
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–14. https://doi.org/10.1007/s11095-014-1593-y.
Li C, Zhang Y, Su T, Feng L, Long Y, Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int J Nanomedicine. 2012;7:5995–6002. https://doi.org/10.2147/IJN.S38043.
Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–36. https://doi.org/10.1208/s12248-012-9444-4.
Li Z, Zheng Z, Ruan J, Li Z, Tzeng CM. Chronic inflammation links cancer and Parkinson’s disease. Front Aging Neurosci. 2016;8:126. https://doi.org/10.3389/fnagi.2016.00126.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–14. https://doi.org/10.1038/mt.2010.105.
Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. https://doi.org/10.4143/crt.2014.46.1.2.
Jager R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of curcumin formulations. Nutr J. 2014;13:11. https://doi.org/10.1186/1475-2891-13-11.
Song Z, Lu Y, Zhang X, Wang H, Han J, Dong C. Novel curcumin-loaded human serum albumin nanoparticles surface functionalized with folate: characterization and in vitro/vivo evaluation. Drug Des Devel Ther. 2016;10:2643–9. https://doi.org/10.2147/DDDT.S112039.
Liskova K, Kelly AL, O'Brien N, Brodkorb A. Effect of denaturation of alpha-lactalbumin on the formation of BAMLET (bovine alpha-lactalbumin made lethal to tumor cells). J Agric Food Chem. 2010;58(7):4421–7. https://doi.org/10.1021/jf903901j.
Hoque M, Dave S, Gupta P, Saleemuddin M. Oleic acid may be the key contributor in the BAMLET-induced erythrocyte hemolysis and tumoricidal action. PLoS One. 2013;8(9):e68390. https://doi.org/10.1371/journal.pone.0068390.
Rath EM, Duff AP, Hakansson AP, Vacher CS, Liu GJ, Knott RB, et al. Structure and potential cellular targets of HAMLET-like anti-cancer compounds made from milk components. J Pharm Pharm Sci. 2015;18(4):773–824.
Rammer P, Groth-Pedersen L, Kirkegaard T, Daugaard M, Rytter A, Szyniarowski P, et al. BAMLET activates a lysosomal cell death program in cancer cells. Mol Cancer Ther. 2010;9(1):24–32. https://doi.org/10.1158/1535-7163.MCT-09-0559.
Acknowledgements
We are thankful to Dr. Manicka V. Vadhanam for useful discussions and help with the animal studies. Curcumin used in this study was generously provided by Sabinsa Corp.
Funding
This work was supported by Agnes Brown Duggan Endowment and Helmsley Trust Funds, awarded to R.C.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals were maintained under a 12-h light/12-h dark cycle in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. All the animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, per the spirit of Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) expectations for animal care and use/ethics.
Additional information
Guest Editors: Juliane Nguyen and Steven Jay
Electronic supplementary material
ESM 1
(DOCX 1814 kb)
Rights and permissions
About this article
Cite this article
Aqil, F., Munagala, R., Jeyabalan, J. et al. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. AAPS J 19, 1691–1702 (2017). https://doi.org/10.1208/s12248-017-0154-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0154-9