Abstract
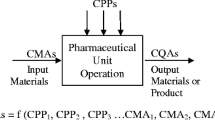
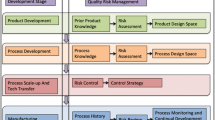
The translation of nanomedicines from concepts to commercial products has not reached its full potential, in part because of the technical and regulatory challenges associated with chemistry, manufacturing, and controls (CMC) development of such complex products. It is critical to take a quality by design (QbD) approach to developing nanomedicines—using a risk-based approach to identifying and classifying product attributes and process parameters and ultimately developing a deep understanding of the products, processes, and platform. This article exemplifies a QbD approach used by BIND Therapeutics, Inc., to industrialize a polymeric targeted nanoparticle drug delivery platform. The focus of the approach is on CMC affairs but consideration is also given to preclinical, clinical, and regulatory aspects of pharmaceutical development. Processes are described for developing a quality target product profile and designing supporting preclinical studies, defining critical quality attributes and process parameters, building a process knowledge map, and employing QbD to support outsourced manufacturing.








Similar content being viewed by others
References
Havel H, Finch G, Strode P, Wolfgang M, Zale S, Bobe I, et al. Nanomedicines: from bench to bedside and beyond. AAPS J. 2016. doi:10.1208/s12248-016-9961-7.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48.
Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010.
Gothwal A, Khan I, Gupta U. Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharm Res. 2016;33(1):18–39.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22.
Tunn UW, Gruca D, Bacher P. Six-month leuprorelin acetate depot formulations in advanced prostate cancer: a clinical evaluation. Clin Interv Aging. 2013;8:457.
Ballav C, Gough S. Bydureon: long‐acting exenatide for once‐weekly injection. Prescriber. 2012;23(1–2):30–3.
Harrison TS, Goa KL. Long-acting risperidone. CNS Drugs. 2004;18(2):113–32.
Syed YY, Keating GM. Extended-release intramuscular naltrexone (VIVITROL®): a review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs. 2013;27(10):851–61.
Stone GW, Teirstein PS, Meredith IT, Farah B, Dubois CL, Feldman RL, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM workhorse trial. J Am Coll Cardiol. 2011;57(16):1700–8.
Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel® wafers in the treatment of malignant glioma: a systematic review. Curr Oncol. 2007;14(5):189–94.
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.
Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39.
Gaddy DF, Lee H, Zheng J, Jaffray DA, Wickham TJ, Hendriks BS. Whole-body organ-level and kidney micro-dosimetric evaluations of 64Cu-loaded HER2/ErbB2-targeted liposomal doxorubicin (64Cu-MM-302) in rodents and primates. EJNMMI Res. 2015;5(1):24.
Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1(7):493–502.
Baselga J, Albanell J. Targeting epidermal growth factor receptor in lung cancer. Curr Oncol Rep. 2002;4(4):317–24.
Ashton S, Song YH, Nolan J, Cadogan E, Murray J, Odedra R, et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med. 2016;8(325):325ra17.
Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318.
Kulkarni SA, Feng SS. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30:2512–22.
Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL, et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52:347–57.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8.
ICH Harmonised Tripartite Guideline: Pharmaceutical Development Q8(R2); ICH Harmonised Tripartite Guideline: Quality Risk Management Q9; ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10.
Product development and realisation case study A-Mab, ISPE CMC Biotech Working Group, 2009.
Luciani F, Galluzzo S, Gaggioli A, Kruse NA, Venneugues P, Schneider CK, et al. MAbs. 2015;7(3):451–5. doi:10.1080/19420862.2015.1023058. Review.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. AAPS J. 2014;16(4):771–83. doi:10.1208/s12248-014-9598-3.
Roudier B, Davit B, Schütz H, Cardot JM. AAPS J. 2015;17(1):24–34. doi:10.1208/s12248-014-9680-x.
Sihem A-O. Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology. Pharmaceutics. 2014;6:137–74.
Song YH, Shin E, Wang NJ, Low S, Zale S. A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system. J Control Release. 2016;229:106–19.
Online Size analysis of nanoparticles | Particle sizing systems http://pssnicomp.com/?page_id=2543
CMC Biotech Working Group. A-Mab: a case study in bioprocess development. October 30, 2009. Available at: www.ispe.org/pqli/a-mab-case-study-version-2.1.
Troiano G, Parsons D, Van Geen Hoven C, Tuller S, Dey J, Brigida L. Selecting and working with CMOs for complex formulations and processes. Pharmaceut Outsour. 2015. Available at: www.pharmoutsourcing.com/Featured-Articles/178208-Selecting-and-Working-with-CMOs-for-Complex-Formulations-and-Processes/.
Liposome drug products guidance for industry, FDA 2015.
Peraman R, Bhadraya K, Padmanabha Reddy Y. Analytical quality by design: a tool for regulatory flexibility and robust analytics. Int J Anal Chem. 2015;(2015);article ID no. 868727:9 pages.
Acknowledgments
We would like to thank Liza Andrianova, Laura Beaudoin, Nicholas Boylan, Jeff Boyle, Lia Brigida, Louise Cadzow, Vicki Campbell, Jessica Cheney, Jagannath Dey, Mike Figa, Allen Horhota, Susan Low, Eric Lewis-Clark, Allison MacRae, Kevin McDonnell, Erick Peeke, Beadle Retnarajan, Abhimanyu Sabnis, Jeffrey Song, Young-Ho Song, Grace Yao, and Jonathan Yingling for the helpful scientific discussion, technical support, and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Katherine Tyner, Sau (Larry) Lee, and Marc Wolfgang
Rights and permissions
About this article
Cite this article
Troiano, G., Nolan, J., Parsons, D. et al. A Quality by Design Approach to Developing and Manufacturing Polymeric Nanoparticle Drug Products. AAPS J 18, 1354–1365 (2016). https://doi.org/10.1208/s12248-016-9969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9969-z