Abstract
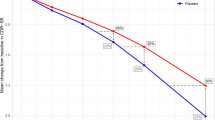
Mixed-effects beta regression (BR), boundary-inflated beta regression (ZOI), and coarsening model (CO) were investigated for analyzing bounded outcome scores with data at the boundaries in the context of Alzheimer’s disease. Monte Carlo simulations were conducted to simulate disability assessment for dementia (DAD) scores using these three models, and each set of simulated data were analyzed by the original simulation model. One thousand trials were simulated, and each trial contained 250 subjects. For each subject, DAD scores were simulated at baseline, 13, 26, 39, 52, 65, and 78 weeks. The simulation-reestimation exercise showed that all the three models could reasonably recover their true parameter values. The bias of the parameter estimates of the ZOI model was generally less than 1%, while the bias of the CO model was mainly within 5%. The bias of the BR model was slightly higher, i.e., less than or in the order of 20%. In the application to real-world DAD data from clinical studies, examination of prediction error and visual predictive check (VPC) plots suggested that both BR and ZOI models had similar predictive performance and described the longitudinal progression of DAD slightly better than the CO model. In conclusion, the investigated three modeling approaches may be sensible choices for bounded outcome scores with data on the edges. Prediction error and VPC plots can be used to identify the model with best predictive performance.

Similar content being viewed by others
REFERENCES
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–64.
Gelinas I et al. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999;53(5):471–81.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Tekin S et al. Activities of daily living in Alzheimer's disease: neuropsychiatric, cognitive, and medical illness influences. Am J Geriatr Psychiatry. 2001;9(1):81–6.
Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–36.
Swearingen CJ, Melguizo Castro MS, Bursac Z. Modeling percentage outcomes: the %beta_regression macro. SAS®Global Forum Proceedings. 2011;335:1–12.
Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods. 2006;11(1):54–71.
Cribari-Neto F, Zeileis A. Beta Regression in R. J Stat Softw. 2010;34(2):1–24.
Ferrari SP, Cribari-Neto F. Beta regression for modelling rates and proportions. J Appl Stat. 2004;31(7):799–815.
Ospina R, Ferrari SP. Inflated beta distributions. Stat Pap. 2010;51:111–26.
Ospina R, Ferrari SP. A general class of zero-or-one inflated beta regression models. Comput Stat Data Anal. 2011;56(6):1609–23.
Ospina R, Ferrari SP. A general class of zero-or-one inflated beta regression models. Comput Stat Data Anal. 2012;56(6):1609–23.
Verkuilen J, Smithson M. Mixed and mixture regression models for continuous bounded responses using the beta distribution. J Educ Behav Stat. 2012;37(1):82–113.
Molas M, Lesaffre E. A comparison of three random effects approaches to analyze repeated bounded outcome scores with an application in a stroke revalidation study. Stat Med. 2008;27(30):6612–33.
Ito K, Hutmacher M, Corrigan B. Modeling of Functional Assessment Questionnaire (FAQ) as continuous bounded data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database [Poster]. American Conference on Pharmacometrics. In American Conference on Pharmacometrics. San Diego; 2011.
Ito K, Hutmacher MM, Corrigan BW. Modeling of Functional Assessment Questionnaire (FAQ) as continuous bounded data from the ADNI database. J Pharmacokinet Pharmacodyn. 2012;39(6):601–18.
Hutmacher MM et al. Estimating transformations for repeated measures modeling of continuous bounded outcome data. Stat Med. 2012;30(9):935–49.
Hu C et al. Bounded outcome score modeling: application to treating psoriasis with ustekinumab. J Pharmacokinet Pharmacodyn. 2011;38(4):497–517.
Ito K et al. Disease progression meta-analysis model in Alzheimer's disease. Alzheimers Dement. 2010;6(1):39–53.
Holford NH, Peace KE. Results and validation of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci U S A. 1992;89(23):11471–5.
Holford NH, Peace KE. Methodologic aspects of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci U S A. 1992;89(23):11466–70.
Kieschnick R, McCullough BD. Regression analysis of variates observed on (0, 1): percentages, proportions and fractions. Stat Model. 2003;3:193–213.
Albert JM, Wang W, Nelson S. Estimating overall exposure effects for zero-inflated regression models with application to dental caries. Stat Methods Med Res. 2011
Feldman H, Sauter A, Donald A, Gelinas I, Gauthier S, Torfs KA, et al. The disability assessment for dementia scale: a 12-month study of functional ability in mild to moderate severity Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(2):89–95.
Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–33.
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–70.
Suh GH, Ju YS, Yeon BK, Shah A. A longitudinal study of Alzheimer's disease: rates of cognitive and functional decline. Int J Geriatr Psychiatr. 2004;19(9):817–24.
Xu XS, Samtani MN, Dunne A, Nandy P, Vermeulen A, Ridder F. Mixed-effects beta regression for modeling continuous bounded outcome scores using NONMEM when data are not on the boundaries. J Pharmacokinet Pharmacodyn. 2013;1–8
ACKNOWLEDGMENTS
There is no conflict of interest. Steven Xu, Mahesh Samtani, and Partha Nandy are employees of Johnson & Johnson Pharmaceutical Research & Development. Min Yuan is an associate professor at University of Science and Technology of China and is partially supported by the National Science Foundation of China (NSFC), grant no. 11201452 and no. 11271346.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 20 kb)
Supplementary Fig. 2
(DOCX 21 kb)
Supplementary Table 1
(DOCX 17.4 kb)
Appendices
APPENDIX 1
APPENDIX 2
APPENDIX 3
APPENDIX 4
Nine hundred (900) random data were sampled from a beta distribution, beta(95, 5). Then, 100 of 1’s were added to the data as boundary data. BR was performed to re-estimate the parameters of the beta distribution based on the combined data after rescaling with a small δ. The density of the original data, rescaled data, and re-estimated distribution are shown in Supplementary Figs. 1 (δ = 1e−8) and 2 (δ = 1e−2).
Rights and permissions
About this article
Cite this article
Xu, X.S., Samtani, M., Yuan, M. et al. Modeling of Bounded Outcome Scores with Data on the Boundaries: Application to Disability Assessment for Dementia Scores in Alzheimer’s Disease. AAPS J 16, 1271–1281 (2014). https://doi.org/10.1208/s12248-014-9655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9655-y