Abstract
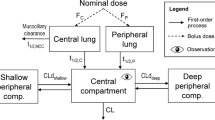
The pharmacokinetic (PK) behavior of inhaled drugs is more complicated than that of other forms of administration. In particular, the effects of certain physiological (mucociliary clearance and differences in membrane properties in central and peripheral (C/P) areas of the lung), formulation (as it relates to drug deposition and particle dissolution rate), and patient-related factors (lung function; effects on C/P deposition ratio) affect the systemic PKs of inhaled drugs. The objectives of this project were (1) to describe a compartmental model that adequately describes the fate of inhaled corticosteroids (ICS) after administration while incorporating variability between and within subjects and (2) based upon the model, to provide a freely available tool for simulation of PK trials after ICS administration. This compartment model allows for mucociliary removal of undissolved particles from the lung, distinguishes between central and peripheral regions of the lung, and models drug entering the systemic circulation via the lung and the gastrointestinal tract. The PK simulation tool is provided as an extension package to the statistical software R (‘ICSpkTS’). It allows simulation of PK trials for hypothetical ICS and of four commercially available ICS (budesonide, flunisolide, fluticasone propionate, and triamcinolone acetonide) in a parallel study design. Simulated PK data and parameters agreed well with literature data for all four ICS. The ICSpkTS package is especially suitable to explore the effect of changes in model parameters on PK behavior and can be easily adjusted for other inhaled drugs.



Similar content being viewed by others
Notes
Other reasons such as variability in systemic distribution micro-constants (k 12 and k 21) across studies cannot be excluded.
REFERENCES
Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/uploads/users/files/GINA_Report_2011.pdf. Accessed 12 June 2012.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Jan21.pdf. Accessed 12 June 2012.
Hochhaus G, Mollmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37:881–92.
Hochhaus G. New developments in corticosteroids. Proc Am Thorac Soc. 2004;1:269–74.
Brutsche MH, Brutsche IC, Munawar M, Langley SJ, Masterson CM, Daley-Yates PT, et al. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356:556–61.
Singh SD, Whale C, Houghton N, Daley-Yates P, Kirby SM, Woodcock AA. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2003;55:375–81.
Harrison LI, Novak CC, Needham MJ, Ratner P. Comparative pulmonary function and pharmacokinetics of fluticasone propionate and salmeterol xinafoate delivered by two dry powder inhalers to patients with asthma. J Aerosol Med Pulm Drug Deliv. 2011;24:245–52.
Holford NH, Kimko HC, Monteleone JP, Peck CC. Simulation of clinical trials. Annu Rev Pharmacol Toxicol. 2000;40:209–34.
Brown Jr RA, Schanker LS. Absorption of aerosolized drugs from the rat lung. Drug Metab Dispos. 1983;11:355–60.
Schanker LS, Mitchell EW, Brown Jr RA. Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab Dispos. 1986;14:79–88.
Edsbacker S, Wollmer P, Selroos O, Borgstrom L, Olsson B, Ingelf J. Do airway clearance mechanisms influence the local and systemic effects of inhaled corticosteroids? Pulm Pharmacol Ther. 2008;21:247–58.
R Development Core Team. R: A language and environment for statistical computing. http://www.r-project.org/. Accessed 12 June 2012.
Wu K, Blomgren AL, Ekholm K, Weber B, Edsbaecker S, Hochhaus G. Budesonide and ciclesonide: effect of tissue binding on pulmonary receptor binding. Drug Metab Dispos. 2009;37:1421–6.
Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci. 1986;75:433–8.
Lee SL, Adams WP, Li BV, Conner DP, Chowdhury BA, Yu LX. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–23.
O’Riordan TG, Zwang J, Smaldone GC. Mucociliary clearance in adult asthma. Am Rev Respir Dis. 1992;146:598–603.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Informa Healthcare; 1982. p. 419–24.
Krishnaswami S, Hochhaus G, Derendorf H. An interactive algorithm for the assessment of cumulative cortisol suppression during inhaled corticosteroid therapy. AAPS PharmSci. 2000;2:28–37.
Clark AR. Understanding penetration index measurements and regional lung targeting. J Aerosol Med Pulm Drug Deliv. 2012;25:179–87.
Moellmann HW, Hochhaus G, Tromm A, Froehlich P, Moellmann AC, Krieg M, et al. Pharmcokinetics and pharmacodynamics of budesonide pH-modified release capsules. In: Moellmann HW, May B, editors. Glucocortocoid therapy in chronic inflammatory bowel disease. Norwell: Kluwer; 1996. p. 107–20.
Derendorf H, Hochhaus G, Rohatagi S, Mollmann H, Barth J, Sourgens H, et al. Pharmacokinetics of triamcinolone acetonide after intravenous, oral, and inhaled administration. J Clin Pharmacol. 1995;35:302–5.
Rohatagi S, Hochhaus G, Mollmann H, Barth J, Galia E, Erdmann M, et al. Pharmacokinetic and pharmacodynamic evaluation of triamcinolone acetonide after intravenous, oral, and inhaled administration. J Clin Pharmacol. 1995;35:1187–93.
Borgstrom L. Deposition patterns with Turbuhaler. J Aerosol Med. 1994;7:S49–53.
Borgstrom L, Bondesson E, Moren F, Trofast E, Newman SP. Lung deposition of budesonide inhaled via Turbuhaler: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994;7:69–73.
Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat Soft Mist inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhaler. J Aerosol Med. 2005;18:264–72.
Wildhaber JH, Devadason SG, Wilson JM, Roller C, Lagana T, Borgstrom L, et al. Lung deposition of budesonide from turbuhaler in asthmatic children. Eur J Pediatr. 1998;157:1017–22.
Ryrfeldt A, Andersson P, Edsbäcker S, Tönnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86–95.
Thorsson L, Edsbacker S, Conradson TB. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44.
Kallen A, Thorsson L. Drug disposition analysis: a comparison between budesonide and fluticasone. J Pharmacokinet Pharmacodyn. 2003;30:239–56.
Mollmann H, Wagner M, Krishnaswami S, Dimova H, Tang Y, Falcoz C, et al. Single-dose and steady-state pharmacokinetic and pharmacodynamic evaluation of therapeutically clinically equivalent doses of inhaled fluticasone propionate and budesonide, given as Diskus or Turbohaler dry-powder inhalers to healthy subjects. J Clin Pharmacol. 2001;41:1329–38.
Thorsson L, Edsbacker S, Kallen A, Lofdahl CG. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52:529–38.
Mackie AE, Ventresca GP, Fuller RW, Bye A. Pharmacokinetics of intravenous fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1996;41:539–42.
Krishnaswami S, Hochhaus G, Mollmann H, Barth J, Derendorf H. Interpretation of absorption rate data for inhaled fluticasone propionate obtained in compartmental pharmacokinetic modeling. Int J Clin Pharmacol Ther. 2005;43:117–22.
Berridge MS, Lee Z, Heald DL. Pulmonary distribution and kinetics of inhaled [11C]triamcinolone acetonide. J Nucl Med. 2000;41:1603–11.
Harrison TW, Tattersfield AE. Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003;58:258–60.
Mortimer KJ, Tattersfield AE, Tang Y, Wu K, Lewis S, Hochhaus G, et al. Plasma concentrations of fluticasone propionate and budesonide following inhalation: effect of induced bronchoconstriction. Br J Clin Pharmacol. 2007;64:439–44.
Dalby C, Polanowski T, Larsson T, Borgstrom L, Edsbacker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10:104.
Argenti D, Shah B, Heald D. A study comparing the clinical pharmacokinetics, pharmacodynamics, and tolerability of triamcinolone acetonide HFA-134a metered-dose inhaler and budesonide dry-powder inhaler following inhalation administration. J Clin Pharmacol. 2000;40:516–26.
Hirst PH, Pitcairn GR, Richards JC, Rohatagi S, Gillen MS, Newman SP. Deposition and pharmacokinetics of an HFA formulation of triamcinolone acetonide delivered by pressurized metered dose inhaler. J Aerosol Med. 2001;14:155–65.
Mollmann H, Derendorf H, Barth J, Meibohm B, Wagner M, Krieg M, et al. Pharmacokinetic/pharmacodynamic evaluation of systemic effects of flunisolide after inhalation. J Clin Pharmacol. 1997;37:893–903.
Nolting A, Sista S, Abramowitz W. Flunisolide HFA vs flunisolide CFC: pharmacokinetic comparison in healthy volunteers. Biopharm Drug Dispos. 2001;22:373–82.
Nolting A, Sista S, Abramowitz W. Single-dose study to compare the pharmacokinetics of HFA flunisolide and CFC flunisolide. J Pharm Sci. 2002;91:424–32.
Richards J, Hirst P, Pitcairn G, Mahashabde S, Abramowitz W, Nolting A, et al. Deposition and pharmacokinetics of flunisolide delivered from pressurized inhalers containing non-CFC and CFC propellants. J Aerosol Med. 2001;14:197–208.
Zaborny BA, Lukacsko P, Barinov-Colligon I, Ziemniak JA. Inhaled corticosteroids in asthma: a dose-proportionality study with triamcinolone acetonide aerosol. J Clin Pharmacol. 1992;32:463–9.
Gonda I. Drugs administered directly into the respiratory tract: modeling of the duration of effective drug levels. J Pharm Sci. 1988;77:340–6.
Hofmann W. Modelling inhaled particle deposition in the human lung—a review. J Aerosol Sci. 2011;42:693–724.
Mortimer KJ, Harrison TW, Tang Y, Wu K, Lewis S, Sahasranaman S, et al. Plasma concentrations of inhaled corticosteroids in relation to airflow obstruction in asthma. J Clin Pharmacol. 2006;62:412–9.
Byron PR, Hindle M, Lange CF, Longest PW, McRobbie D, Oldham MJ, et al. In vivo–in vitro correlations: predicting pulmonary drug deposition from pharmaceutical aerosols. J Aerosol Med Pulm Drug Deliv. 2010;23 Suppl 2:S59–69.
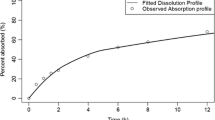
Son Y-J, Horng M, Copley M, McConville JT. Optimization of an in vitro dissolution test method for inhalation formulations. Dissolution Technologies. 2010;1–8:6–13.
Rohatagi S, Bye A, Mackie AE, Derendorf H. Mathematical modeling of cortisol circadian rhythm and cortisol suppression. Eur J Pharm Sci. 1996;4:341–50.
ACKNOWLEDGMENTS
We would like to thank Saskia Fuhrmann, Uta Schilling, and especially Bhargava Kandala, for all their assistance with this project.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix
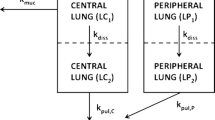
Laplace transformation (17) of Eqs. 1–7 and rearrangements yields
where s represents the Laplace operator, F BA was defined above, and LC1,0, LP1,0, and A 0 denote the initial amount of drug in the dissolution compartments of the central and peripheral lung, and the GI absorption compartment, respectively. Particularly,
where Dose is the by-the-inhaler-emitted dose, F Lung is the fraction of the emitted dose that is deposited in the lung, and F C is the fraction of the lung dose that is deposited in central lung regions. Substitution of Eq. 10–14 and Eq. 16 into Eq. 15 and defining α * β = k 10 * k 21 and α + β = k 10 + k 12 + k 21 yields
where
Anti Laplace transformation (17) of Eq. 20 yields
where
and
ICSpkTS R EXTENSION PACKAGE—HANDS-ON EXAMPLES
The structure and functions of the ICSpkTS extension package are briefly explained in form of two hands-on examples. In the first example, the ICS module of the ICSpkTS package is introduced by a case study comparing the AUC and C max in healthy subjects and asthmatic patients. In the second example, the effect of having two FP formulations that differ in their pulmonary dissolution rate constant on the PK behavior is used to explain the FP module. Further details and functions of the ICSpkTS package can be found in the official documentation (available via http://www.cop.ufl.edu/pc/research/areas-of-research/inhaled-glucocorticoids/icspkts-r-extension/) and/or by accessing the official R help files.
HANDS-ON EXAMPLE 1: ICS
Running the following code in R will simulate a PK trial when 500 mcg of an hypothetical ICS are administered to 25 subjects per treatment group (healthy subjects (situation A) vs. asthmatic patients (situation B)) and plasma samples are obtained at 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h after administration. The asthmatic patients are modeled by increasing the fraction of drug that is deposited in the central regions of the lung and by lowering the mucociliary clearance rate constant.
#####################################################
#Number of Subjects (n) per Group
#####################################################
n.subjects = 25
#####################################################
#Time points (h) where plasma samples are obtained
#####################################################
Time = c(0.17,0.33,0.5,1,1.5,2,3,4,6,8,10,12,14,16,18,20,22,24)
#####################################################
#Situation A-Model Parameters-Typical Values (TV) and
#Between-Subject Variability (BSV)
#####################################################
Dose.A = 500
TV.FLung.A = 0.2
TV.FC.A = 0.5
TV.FBA.A = 0.1
TV.kdiss.A = 0.3
TV.kmuc.A = 0.5
TV.kpulC.A = 0.4
TV.kpulP.A = 0.4
TV.ka.A = 0.65
TV.CL.A = 49
TV.VC.A = 87
TV.k12.A = 0.1
TV.k21.A = 0.05
BSV.FLung.A = 0.2
BSV.FC.A = 0.2
BSV.FBA.A = 0.2
BSV.kdiss.A = 0.2
BSV.kmuc.A = 0.2
BSV.kpulC.A = 0.2
BSV.kpulP.A = 0.2
BSV.ka.A = 0.2
BSV.CL.A = 0.2
BSV.VC.A = 0.2
BSV.k12.A = 0.2
BSV.k21.A = 0.2
#####################################################
#Situation B-Model Parameters-Typical Values (TV) and
#Between-Subject Variability (BSV)
#####################################################
Dose.B = 500
TV.FLung.B = 0.2
TV.FC.B = 0.8
TV.FBA.B = 0.1
TV.kdiss.B = 0.3
TV.kmuc.B = 0.25
TV.kpulC.B = 0.4
TV.kpulP.B = 0.4
TV.ka.B = 0.65
TV.CL.B = 49
TV.VC.B = 87
TV.k12.B = 0.1
TV.k21.B = 0.05
BSV.FLung.B = 0.2
BSV.FC.B = 0.2
BSV.FBA.B = 0.2
BSV.kdiss.B = 0.2
BSV.kmuc.B = 0.2
BSV.kpulC.B = 0.2
BSV.kpulP.B = 0.2
BSV.ka.B = 0.2
BSV.CL.B = 0.2
BSV.VC.B = 0.2
BSV.k12.B = 0.2
BSV.k21.B = 0.2
#####################################################
#Within Subject Variability (WSV)
#####################################################
WSV = 0.3
#####################################################
#PK Trial Simulation
#####################################################
ICS(plots = FALSE,tables = FALSE)
The following output displaying the AUC and C max for both healthy subjects and asthmatic patients and 90% confidence intervals of the geometric means ratios (healthy/asthmatic) for both AUC and C max is generated by the ICSpkTS package.
Simulation was successful
AUC-Means (Arithmetic Means):
Situation A-Situation B
[1] 2.23 1.96
Cmax-Means (Arithmetic Means):
Situation A-Situation B
[1] 0.43 0.34
AUC-90% Confidence Interval (Geometric Mean Ratio):
[1] 0.99 1.28
Cmax-90% Confidence Interval (Geometric Mean Ratio):
[1] 1.04 1.39
Furthermore, a graph showing the average plasma concentration time profiles for both healthy subjects and asthmatic patients is created (Fig. 4).
Hands-on example 1, ICS module, simulation of a PK trial after administration of 500 mcg of a hypothetical ICS to 25 subjects per treatment group (healthy subjects (situation A) vs. asthmatic patients (situation B)), and plasma samples are obtained at 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h after administration. Asthmatic patients are modeled by increasing the fraction of drug that is deposited in the central regions of the lung and by lowering the mucociliary clearance rate constant
HANDS-ON EXAMPLE 2: FP
Running the following code in R will simulate a PK trial when 500 mcg FP are administered to 35 subjects per formulation group (formulation A and B differ in their dissolution rate constants, B dissolves 3-fold faster) and plasma samples are obtained at 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 20, and 24 h after administration.
#####################################################
#Number of Subjects (n) per Group
#####################################################
n.subjects = 35
#####################################################
#Time points (h) where plasma samples are obtained
#####################################################
Time = c(0.17,0.33,0.5,1,1.5,2,3,4,6,8,10,12,16,20,24)
#####################################################
#Formulation A-Model Parameters-Typical Values (TV) # and Between-Subject Variability (BSV)
#####################################################
Dose.A = 500
TV.FLung.A = 0.16
TV.FC.A = 0.5
TV.kdiss.A = 0.302
TV.kmuc.A = 0.938
TV.kpulC.A = 10
TV.kpulP.A = 20
BSV.FLung.A = 0.2
BSV.FC.A = 0.2
BSV.kdiss.A = 0.2
BSV.kmuc.A = 0.2
BSV.kpulC.A = 0.2
BSV.kpulP.A = 0.2
BSV.CL.A = 0.2
BSV.VC.A = 0.2
BSV.k12.A = 0.2
BSV.k21.A = 0.2
#####################################################
#Formulation B-Model Parameters-Typical Values (TV) and
#Between-Subject Variability (BSV)
#####################################################
Dose.B = 500
TV.FLung.B = 0.16
TV.FC.B = 0.5
TV.kdiss.B = 0.9
TV.kmuc.B = 0.938
TV.kpulC.B = 10
TV.kpulP.B = 20
BSV.FLung.B = 0.2
BSV.FC.B = 0.2
BSV.kdiss.B = 0.2
BSV.kmuc.B = 0.2
BSV.kpulC.B = 0.2
BSV.kpulP.B = 0.2
BSV.CL.B = 0.2
BSV.VC.B = 0.2
BSV.k12.B = 0.2
BSV.k21.B = 0.2
#####################################################
#Within-Subject Variability (WSV)
#####################################################
WSV = 0.3
#####################################################
#PK Trial Simulation
#####################################################
FP(plots = FALSE,tables = FALSE)
The following output displaying the AUC and C max for both formulations and 90% confidence intervals of the geometric means ratios (A/B) for both AUC and C max is generated by the ICSpkTS package.
Simulation was successful
AUC-Means (Arithmetic Means):
Formulation A-Formulation B
[1] 0.71 0.73
Cmax-Means (Arithmetic Means):
Formulation A-Formulation B
[1] 0.20 0.38
AUC-90% Confidence Interval (Geometric Mean Ratio):
[1] 0.85 1.09
Cmax-90% Confidence Interval (Geometric Mean Ratio):
[1] 0.45 0.60
Moreover, a graph showing the average plasma concentration time profiles for both formulations is generated (Fig. 5).
Hands-on example 2, FP module, simulation of a PK trial when 500 mcg FP are administered to 35 subjects per formulation group (formulations A and B differ in their dissolution rate constants; B dissolves 3-fold faster), and plasma samples are obtained at 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 20, and 24 h after administration
Rights and permissions
About this article
Cite this article
Weber, B., Hochhaus, G. A Pharmacokinetic Simulation Tool for Inhaled Corticosteroids. AAPS J 15, 159–171 (2013). https://doi.org/10.1208/s12248-012-9420-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9420-z