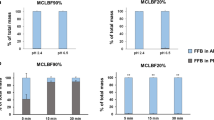
Abstract
With the increasing number of poorly water-soluble compounds in contemporary drug discovery pipelines, the concept of supersaturation as an effective formulation approach for enhancing bioavailability is gaining momentum. This is intended to design the formulation to yield significantly high intraluminal concentrations of the drug than the thermodynamic equilibrium solubility through achieving supersaturation and thus to enhance the intestinal absorption. The major challenges faced by scientists developing supersaturatable formulations include controlling the rate and degree of supersaturation with the application of polymeric precipitation inhibitor and maintenance of post-administration supersaturation. This review is intended to cover publications on this topic since April 2009. Scientific publications associated with characterization of supersaturatable systems and related preclinical and clinical pharmacokinetics (PK) studies are reviewed. Specifically, this review will address issues related to assessing the performance of supersaturatable systems including: (1) Diversified approaches for developing supersaturatable formulations, (2) meaningful in vitro test methods to evaluate supersaturatable formulations, and (3) in vivo PK study cases which have demonstrated direct relevance between the supersaturation state and the exposure observed in animal models and human subjects.









Similar content being viewed by others
REFERENCES
Gao P, Morozowich W. Design and development of a new class of supersaturatable SEDDS with potential for enhanced oral absorption and reduced GI side effects. In: Hauss DJ, editor. Lipid based formulations for oral drug delivery: enhancing the bioavailability of poorly water-soluble drugs. New York: Dekker; 2007.
Gao P, Morozowich W. Development of supersaturatable SEDDS (S-SEDDS) formulations for improving the oral absorption of poorly soluble drugs. Exp Opin Drug Deliv. 2005;3:97–110.
Brouwwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Mcallister M. Dynamic dissolution: a step closer to predicitive dissolution testing? Mol Pharm. 2010;7(5):1374–87.
Mellaerts R, Aerts A, Caremans TP, Vermant J, Van den Mooter G, Martens JA, Augustijns P. Growth of itraconazole nanofibers in supersaturated simulated intestinal fluid. Mol Pharm. 2011;7(3):905–13.
Carino SR, Sperry DC, Hawley M. Relative bioavailability estimation of carbamazepine crystal forms using an artificial stomach-duodenum model. J Pharm Sci. 2006;95(1):116–25.
Coutant C, Polster C. Novel dissolution methodologies: FBRM and artificial stomach duodenum. AAPS Webinar. Jan 25, 2012.
Gao Y, Carr RA, Spence JK, Wang W, Turner TM, Lipari JL, Miller JM. A pH-dilution method for estimation of viorelevant drug solubility along the gastrointestinal tract: application to physiologically based pharmacokinetic modeling. Mol Pharm. 2010;7(5):1526–6.
Shi Y, Gao P, Gong Y, Ping H. Application of a biphasic test for characterization of in vitro drug release of immediate release formulations of celecoxib and its relevance to in vivo absorption. Mol Pharm. 2011;7(5):1458–65.
Vangani S, Li X, Zhou P, Del-Barrio M, Chiu R, Cauchon N, Gao P, Medina C, Jasti B. Dissolution of poorly water-soluble drugs in biphasic media using USP 4 and fiber optic system. Clin Res Regul Aff. 2009;26(1–2):8–19.
Buch P, Langguth P, Kataoka M, Yamashita S. IVIVC in oral absorption for fenofibrate immediate release tablets using a dissolution/permeation system. J Pharm Sci. 2009;98(6):2001–9.
Zhang N, Zhang W, Jin Y, Quan DQ. Studies on preparation of carboamazepine (CBZ) supersaturatable self-microemulsifying (S-SMEDDS) formulation and relative bioavailability in beagle dogs. Pharm Dev Technol. 2011;16(4):415–21.
Matteucci ME, Paguio JC, Miller MA, Williams RO, Johnson KP. Highly supersaturated solutions from dissolution of amorphous itraconazole microparticles at pH 6.8. Mol Pharm. 2009;6(2):375–85.
Curatolo W, Nightingale JA, Herbig SM. Utility of hydroxypropylmethylcellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI millirm. Pharm Res. 2009;26:1419–31.
Bevernage J, Forier T, Brouwers J, Tack J, Annaert P, Augustijns P. Excipient-meidated supersaturation stabilization in human intestinal fluids. Mol Pharm. 2011;8:564–70.
Warren DB, Benameur H, Porter CJH, Pouton CW. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target. 2010;18(10):704–31.
DiNunzio JC, Hughey JR, Brough C, Miller DA, Williams RO, McGinity JW. Production of advanced solid dispersions for enhanced bioavailability of itraconazole using KinetiSol dispersing. Drug Dev Ind Pharm. 2010;36(9):1064–78.
Hawley M, Morozowich W. Modifying the diffusion layer of soluble salts of poorly water soluble basic drugs to improve dissolution performance. Mol Pharm. 2010;7(5):1441–9.
Alonzo DE, Zhang GZ, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2009;27(4):608–18.
Alonzo DE, Gao Y, Zhou DL, Mo HP, Zhang GZ, Taylor LS. Dissolution and precipitation behavior of amorphous solid dispersions. J Pharm Sci. 2011;100:3316–31.
Ilevbare GA, Liu HY, Edgar KJ, Taylor LS. Understanding polymer properties important for crystal growth inhibition impact of chemically diverse polymers on solution crystal growth of ritonavir. Cryst Growth Des. 2012;12(6):3133–43.
Patel DD, Joguparthi V, Wang Z, Anderson BD. Maintenance of supersaturation I: indomethacin crystal growth kinetic modeling using an online second-derivative ultraviolet spectroscopic method. J Pharm Sci. 2011;100(7):2623–41.
Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409:260–8.
Van Speybroeck M, Mellaerts R, Mols R, Thi TD, Martens JA, Van Humbeeck J, Annaert P, Van den Mooter G, Augustijns P. Enhanced absorption of the poorly soluble drug fenofibrate by tuning its release rate from ordered mesoporous silica. Eur J Pharm Sci. 2010;41:623–30.
Miller MA, DiNunzio J, Matteucci ME, Ludhe BS, Willimas RO, Johnston KP. Flocculated amorphous itraconazole nanoparticls for enhanced in vitro supersaturation and in vivo bioavailability. Drug Dev Ind Pharm. 2012;38(5):557–70.
Ranzani LS, Font J, Galimany F, Santanach A, Gomez-Gomar AM, Casadevall G, Gryczke A. Enhanced in vivo absorption of CB-1 antagonist in rats via solid solutuions prepared by hot-melt extrusion. Drug Dev Ind Pharm. 2011;37(6):694–701.
Takano R, Takata N, Salto R, Furumoto K, Higo S, Hayashi Y, Machida M, Aso Y, Yamashita S. Quantitative analysis of the effect of supersaturation on in vivo drug absorption. Mol Pharm. 2010;7(5):1431–40.
Polster CS, Atassi F, Wu SJ, Sperry DC. Use of artificial stomach-duodenum model for investigation of dosing fluid effect on clinical trial variability. Mol Pharm. 2010;7(5):1533–8.
Mitra A, Kesisoglou F, Beauchamp M, Zhu W, Chiti F, Wu Y. Using absorption simulation and gastric pH modulated dog model for formulation development to overcome achlorhydria effect. Mol Pharm. 2011;8:2216–23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Ping Gao and Lawrence Yu
Rights and permissions
About this article
Cite this article
Gao, P., Shi, Y. Characterization of Supersaturatable Formulations for Improved Absorption of Poorly Soluble Drugs. AAPS J 14, 703–713 (2012). https://doi.org/10.1208/s12248-012-9389-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9389-7