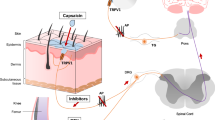
Abstract
In spite of intense research efforts and after the dedicated Decade of Pain Control and Research, there are not many alternatives to opioid-based narcotic analgesics in the therapeutic armamentarium to treat chronic pain conditions. Chronic opioid treatment is associated with sedation, tolerance, dependence, hyperalgesia, respiratory depression, and constipation. Since the affective component is an integral part of pain perception, perhaps it is inevitable that potent analgesics possess the property of impacting pain pathways in the supraspinal structures. The question still remains to be answered is that whether a powerful analgesic can be devoid of narcotic effect and addictive potentials. Local anesthetics are powerful analgesics for acute pain by blocking voltage-gated sodium channels that are involved in generation and propagation of action potentials. Antidepressants and anticonvulsants have proven to be useful in the treatment of certain modalities of pain. In neuropathic pain conditions, the complexity arises because of the notion that neuronal circuitry is altered, as occurs in phantom pain, in that pain is perceived even in the absence of peripheral nociceptive inputs. If the locus of these changes is in the central nervous system, commonly used analgesics may not be very useful. This review focuses on the recent advances in nociceptive transmission and nociceptive transient receptor potential vanilloid 1 channel as a target for treating chronic pain conditions with its agonists/antagonists.
Similar content being viewed by others
References
Cortright DN, Szallasi A. TRP channels and pain. Curr Pharm Des. 2009;15:1736.
Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68.
Khairatkar-Joshi N, Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med. 2009;15:14–21.
Basbaum AI, Jessell TM. Principles of neural science. New York: McGraw-Hill; 2001.
Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30.
Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36.
Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14:175–81.
Ikeda H, Kiritoshi T, Murase K. Synaptic plasticity in the spinal dorsal horn. Neurosci Res. 2009;64:133–6.
Zhao ZQ et al. Cellular basis of itch sensation. Science. 2009;325:1531–4.
Jeffry JA et al. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS ONE. 2009;4:7021.
Scherrer G et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59.
Sherrington C. The integrative action of the nervous system. New York: Scribner; 1906.
Nilius B et al. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:167–217.
Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–62.
Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1–43.
Zeilhofer HU. Synaptic modulation in pain pathways. Rev Physiol Biochem Pharmacol. 2005;154:73–100.
Starowicz K et al. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci. 2007;27(50):13739–49.
Morgan MM, Fields HL. Pronounced changes in the activity of nociceptive modulatory neurons in the rostral ventromedial medulla in response to prolonged thermal noxious stimuli. J Neurophysiol. 1994;72:1161–70.
Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9.
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10.
Nordin M et al. Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain. 1984;20:231–45.
Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage sensitive sodium channels. J Neurophysiol. 1994;72:349–59.
Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–44.
McDonald DM et al. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–62.
Moreau ME et al. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38.
Zahner MR et al. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–23.
Seyedi N, Maruyama R, Levi R. Bradykinin activates a cross-signaling pathway between sensory and adrenergic nerve endings in the heart: a novel mechanism of ischemic norepinephrine release? J Pharmacol Exp Ther. 1999;290:656–63.
Kawamata M, Omote K. Involvement of increased excitatory amino acids and intracellular Ca2+ concentration in the spinal dorsal horn in an animal model of neuropathic pain. Pain. 1996;68:85–96.
Stiller CO et al. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–74.
Pernía-Andrade AJ et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–4.
Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10.
Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–23.
Ahmadi S et al. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40.
Coull JA et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21.
Allan SM, Tyrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40.
Lissitsyn Y et al. Level of Toll-like receptor agonist exposure differentially determines chemokine production in humans. Can J Physiol Pharmacol. 2007;85:739–46.
Pabbidi RM et al. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;1:4–9.
Schilling T, Eder C. Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. J Neuroimmunol. 2009;216:118–21.
Malan TP et al. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol. 2003;3:62–7.
Stucky CL, Gold MS, Zhang X. Mechanisms of pain. Proc Natl Acad Sci U S A. 2001;98:11845–6.
Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109:57–77.
Reuben SS. Preventing the development of complex regional pain syndrome after surgery. Anesthesiology. 2004;101:1215–24.
DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manage Nurs. 2007;8:113–21.
Quirion R. Pain, nociception and spinal opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:571–9.
Mansour A et al. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–9.
Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67–70.
Ross JR et al. Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J. 2005;5:324–36.
Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–91.
Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87.
Vanderah TW et al. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–86.
Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13–20.
Takeda S et al. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95:740–9.
Luca AD, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol Ther. 1996;69:103–15.
Burnstock G. P2X receptors in sensory neurons. Br J Anaesth. 2000;84:476–88.
Sutherland SP et al. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA. 2001;98:711–6.
Ditting T et al. Putative role of epithelial sodium channels (ENaC) in the afferent limb of cardio renal reflexes in rats. Basic Res Cardiol. 2003;98:388–400.
Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Neuron. 2001;32:1097–106.
Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212.
Szallasi A et al. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–72.
Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochem Biophys Acta. 2007;1772:989–1003.
Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann N Y Acad Sci. 2008;1144:42–52.
Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60:2–23.
Gottlieb P et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008;455:1097–103.
Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61:30–44.
Premkumar LS, Sikand P. TRPV1: a target for next generation analgesics. Curr Pharmacol. 2008;6:151–63.
Caterina MJ et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24.
Davis JB et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–7.
Caterina MJ et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13.
Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215–24.
Razavi R et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–35.
Gram DX et al. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in Zucker diabetic fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur J Pharmacol. 2005;509:211–7.
Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118:337–51.
Kohler R et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–502.
O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203.
Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009;583:754–8.
Cao DS, Premkumar LS. Activation of TRPV4 by direct mechanical force. Neurosci Abst. 2008.
Hartmannsgruber V et al. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE. 2007;2:e827.
Tabuchi K et al. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005;382:304–8.
Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–33.
Bandell M et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–57.
Bautista DM et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–52.
Hinman A et al. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–8.
Macpherson LJ et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–5.
Story GM. The emerging role of TRP channels in mechanisms of temperature and pain sensation. Curr Neuropharmacol. 2006;4:183–96.
Bevan S, Andersson DA. TRP channel antagonists for pain—opportunities beyond TRPV1. Curr Opin Investig Drugs. 2009;10:655–63.
Tracey WD et al. Painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–73.
Andersson DA et al. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–94.
Kwan KY et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–89.
Bautista DM et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82.
Obata K et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–401.
Katsura H et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–23.
Kerstein PC, del CD, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19.
Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–19.
Karai L et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–52.
Brown DC et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–9.
Apostolidis A et al. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–5.
Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–7.
Immke DC, Gavva NR. The TRPV1 receptor and nociception. Semin Cell Dev Biol. 2006;17:582–91.
El Kouhen R et al. A- 425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J Pharmacol Exp Ther. 2005;314:400–9.
Acknowledgements
I thank Lauren Hughes and Dr. Mahendra Bishnoi for their valuable comments and for the help with editing of the manuscript. This work was supported by grants from National Institutes of Health (NS042296, DK065742, and DA028017) and EAM award from SIUSOM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Rao Rapaka, Thomas Aigner, Joni Rutter, and David Shurtleff
Rights and permissions
About this article
Cite this article
Premkumar, L.S. Targeting TRPV1 as an Alternative Approach to Narcotic Analgesics to Treat Chronic Pain Conditions. AAPS J 12, 361–370 (2010). https://doi.org/10.1208/s12248-010-9196-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-010-9196-y