Abstract
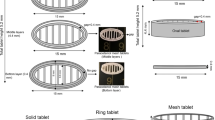
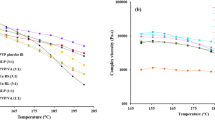
The aims of the current study were to develop and evaluate clindamycin palmitate hydrochloride (CPH) 3D-printed tablets (printlets) manufactured by selective laser sintering (SLS). Optimization of the formulation was performed by studying the effect of formulation and process factors on critical quality attributes of the printlets. The independent factors studied were laser scanning speed, microcrystalline cellulose (MCC), and lactose monohydrate (LMH) concentration. The responses measured were printlets weight, hardness, disintegration time (DT), and dissolution in 30 min. The printlets were characterized for content uniformity, chemical interactions, crystallinity, drug distribution, morphology, and porosity. The laser scanning speed showed statistically significant effects on all the studied dependent responses (p < 0.05). MCC showed statistically significant effects on hardness, DT, and dissolution (p < 0.05), while LMH showed statistically significant effect on hardness and dissolution (p < 0.05). The model was validated by an independent formulation, and empirical values were in close agreement with model-predicted values. X-ray powder diffraction and differential scanning calorimetry data suggested a decrease in crystallinity of the LMH in the printlets. X-ray micro-CT scanning showed porous microstructure of the printlets with a porosity 24.4% and 31.1% for the printlets printed at 200 and 300 mm/s laser speed, respectively. In summary, the SLS method provides an opportunity to fabricate customized dosage forms as per patients’ need.












Similar content being viewed by others
References
Goyanes A, Scarpa M, Kamlow M, Gaisford S, Basit AW, Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm. 2017;530(1–2):71–8. https://doi.org/10.1016/j.ijpharm.2017.07.064.
Sandler N, Preis M. Printed drug-delivery systems for improved patient treatment. Trends Pharmacol Sci. 2017;38(3):317. https://doi.org/10.1016/j.tips.2017.01.002.
United States Food and Drug Administration, Paving the Way for Personalized Medicine: FDA's Role in a New Era of Medical Product -+0Development, 2013. Available from: https://www.fdanews.com/ext/resources/files/10/10-28-13-Personalized-Medicine.pdf.
Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D printing in pharmaceutical and medical applications - recent achievements and challenges. Pharm Res. 2018;35(9):176. https://doi.org/10.1007/s11095-018-2454-x.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–50. https://doi.org/10.1016/j.ijpharm.2015.07.067.
Park K. 3D printing of 5-drug polypill. J Control Release. 2015;217:352. https://doi.org/10.1016/j.jconrel.2015.10.014.
Robles-Martinez P, Xu X, Trenfield SJ, Awad A, Goyanes A, Telford R, et al. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics. 2019;11(6):274. https://doi.org/10.3390/pharmaceutics11060274.
Rahman Z, Barakh Ali SF, Ozkan T, Charoo NA, Reddy IK, Khan MA. Additive manufacturing with 3D printing: progress from bench to bedside. AAPS J. 2018;20(6):101. https://doi.org/10.1208/s12248-018-0225-6.
Rahman Z, Charoo NA, Kuttolamadom M, Asadi A, Khan MA. Printing of personalized medication using binder jetting 3D printer. In: Precision medicine for investigators, practitioners and providers, Edited by Faintuch J, Faintuch S. Academic Press. 2020; 473–481. https://doi.org/10.1016/B978-0-12-819178-1.00046-0.
Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Kadri NA, et al. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16(3):033502. https://doi.org/10.1088/1468-6996/16/3/033502.
Maher S, Kaur G, Lima-Marques L, Evdokiou A, Losic D. Engineering of micro- to nanostructured 3D-printed drug-releasing titanium implants for enhanced osseointegration and localized delivery of anticancer drugs. ACS Appl Mater Interfaces. 2017;9:29562–70.
Barakh Ali SF, Mohamed EM, Ozkan T, Kuttolamadom M, Khan M, Asadi A, et al. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int J Pharm. 2019;570:11865. https://doi.org/10.1016/j.ijpharm.2019.118651.
Awad A, Fina F, Trenfield SJ, Patel P, Goyanes A, Gaisford S, et al. 3D printed pellets (miniprintlets): a novel, multi-drug, controlled release platform technology. Pharmaceutics. 2019;11(4):148. https://doi.org/10.3390/pharmaceutics11040148.
Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm. 2017;529(1–2):285–93. https://doi.org/10.1016/j.ijpharm.2017.06.082.
Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm. 2018;541(1–2):101–7. https://doi.org/10.1016/j.ijpharm.2018.02.015.
Goyanes A, Fina F, Martorana A, Sedough D, Gaisford S, Basit AW. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int J Pharm. 2017b;527(1–2):21–30. https://doi.org/10.1016/j.ijpharm.2017.05.021.
Vithani K, Goyanes A, Jannin V, Basit AW, Gaisford S, Boyd BJ. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm Res. 2018;36(1):4. https://doi.org/10.1007/s11095-018-2531-1.
Rahman Z, Siddiqui A, Khan MA. Orally disintegrating tablet of novel salt of antiepileptic drug: formulation strategy and evaluation. Eur J Pharm Biopharm. 2013;85(3):1300–9. https://doi.org/10.1016/j.ejpb.2013.06.006.
Rahman Z, Xu X, Katragadda U, Krishnaiah YS, Yu L, Khan MA. Quality by design approach for understanding the critical quality attributes of cyclosporine ophthalmic emulsion. Mol Pharm. 2014;11(3):787–99. https://doi.org/10.1021/mp400484g.
Salmoria G, Vieira E, Ghizoni F, Marques MS, Kanis LA. 3D printing of PCL/Fluorouracil tablets by selective laser sintering: properties of implantable drug delivery for cartilage cancer treatment. Rheumatol Orthop Med. 2017;2:1–7. https://doi.org/10.15761/ROM.1000121.
Mazur D, Schug BS, Evers G, Larsimont V, Fieger-Büschges H, Gimbel W, et al. Bioavailability and selected pharmacokinetic parameters of clindamycin hydrochloride after administration of a new 600 mg tablet formulation. Int J Clin Pharmcol Ther. 1999;37(8):386–92.
Cantor SL, Khan MA, Gupta A. Development and optimization of taste-masked orally disintegrating tablets (ODTs) of clindamycin hydrochloride. Drug Dev Ind Pharm 2014; 41(7):1156–1164; doi: 10.3109/03639045 .2014.935392.
Karaman R. Prodrugs for masking the bitter taste of drugs. In: Sezer AD, editor. Application of nanotechnology in drug delivery. London: IntechOpen, InTech; 2014. p. 399–445. https://doi.org/10.5772/58404.
Forist R, DeHaan M, Metzler CM. Clindamycin Bioavailability from clindamycin-2-palmitate and clindamycin-2-hexadecylcarbonate in man. J Pharmacokinet Biopharm. 1973;1(2):89–98. https://doi.org/10.1007/BF01059623.
Cimbollek M, Nies B, Liebendorfer A, Wenz R, Kreuter J. The potential of the prodrug clindamycin palmitate as an implantable slow release form of the antibiotic clindamycin for heart valves. J Control Release. 1995;33(1):47–53. https://doi.org/10.1016/0168-3659(94)00061-X.
Coupland JN, Hayes JE. Physical approaches to masking bitter taste: lessons from food and pharmaceuticals. Pharm Res. 2014;31(11):2921–39. https://doi.org/10.1007/s11095-014-1480-6.
https://www.fda.gov/industry/color-additive-inventories/color-additive-status-list. Accessed on 13th October 2019.
Sangshetti JN, Deshpande M, Zaheer Z, Shinde DB, Arote R. Quality by design approach: regulatory need. Arab J Chem. 2017;10(2):3412–25. https://doi.org/10.1016/j.arabjc.2014.01.025.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83. https://doi.org/10.1208/s12248-014-9598-3.
Tak JW, Gupta B, Thapa RK, Woo KB, Kim SY, Go TG, et al. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box–Behnken design. AAPS PharmSciTech. 2017;18(4):1125–34. https://doi.org/10.1208/s12249-016-0580-5.
Hamilton SJ, Lodder RA. Hyperspectral imaging technology for pharmaceutical analysis, Proc. SPIE 4626. Biomedical Nanotechnology Architectures and Applications. 2002; 136–147. https://doi.org/10.1117/12.472076.
Wu G, Gupta A, Khan M, Faustino P. Development and application of a validated HPLC method for the determination of clindamycin palmitate hydrochloride in marketed drug products: an optimization of the current USP methodology for assay. J Anal Sci Methods Instrum. 2013;3(4):202–11. https://doi.org/10.4236/jasmi.2013.34026.
Mohamed AI, Abd-Motagaly AM, Ahmed OA, Amin S, Mohamed Ali AI. Investigation of drug-polymer compatibility using chemometric-assisted UV spectrophotometry. Pharmaceutics. 2017;9(1):7. https://doi.org/10.3390/pharmaceutics9010007.
Tamaddon L, Mostafavi SA, Karkhane R, Riazi-Esfahani M, Dorkoosh FA, Rafiee-Tehrani M. Thermoanalytical characterization of clindamycin-loaded intravitreal implants prepared by hot melt extrusion. Adv Biomed Res. 2015;4:147. https://doi.org/10.4103/2277-9175.161563.
Akilesh M, Elango PR, Devanand AA, Soundararajan R, Varthanan PA. Optimization of selective laser sintering process parameters on surface quality. In: 3D printing and additive manufacturing technologies; Kumar, L., Pandey, P., Wimpenny, D., Eds; Springer: Singapore. 2019; 141–157; doi: https://doi.org/10.1007/978-981-13-0305-0_13.
Charoo NA, Barakh Ali SF, Mohamed EM, Kuttolamadom M, Ozkan T, Khan M, et al. Selective laser sintering 3D printing – an overview of the technology and pharmaceutical applications. Drug Dev Ind Pharm. 2020;46(6):1–26. https://doi.org/10.1080/03639045.2020.1764027.
Jeon T, Hwang T, Yun H, VanTyne C, Moon YH. Control of porosity in parts produced by a direct laser melting process. Appl Sci. 2018;8(12):2573. https://doi.org/10.3390/app8122573.
Rahman Z, Zidan AS, Habib MJ, Khan MA. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett-Burman design. Int J Pharm. 2010;389(1–2):186–94. https://doi.org/10.1016/j.ijpharm.2009.12.040.
Ali S, Langley N. Formulation - excipients | dry granulation simplifies tableting process. Pharm Formul Q. 2010;3.
Liu H, Wang K, Schlindwein W, Li M. Using the Box–Behnken experimental design to optimise operating parameters in pulsed spray fluidised bed granulation. Int J Pharm. 2013;448(2):329–38. https://doi.org/10.1016/j.ijpharm.2013.03.057.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, E.M., Barakh Ali, S.F., Rahman, Z. et al. Formulation Optimization of Selective Laser Sintering 3D-Printed Tablets of Clindamycin Palmitate Hydrochloride by Response Surface Methodology. AAPS PharmSciTech 21, 232 (2020). https://doi.org/10.1208/s12249-020-01775-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01775-0